Polyelectrolyte Membrane Enables Highly Reversible Zinc Battery Chemistry via Immobilizing Anion and Stabilizing Water
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The integration of water-based electrolytes into zinc-ion batteries encounters challenges due to the limited voltage window of water, interfacial side reactions of mobile counterions, and the growth of zinc metal (Zn0) dendrites during charge. In this study, we introduce a nonfluorinated, cation-conducting polyelectrolyte membrane (PEM) designed to alleviate these challenges by suppressing the reactivities of both water and counterions. This PEM forms hydrogen bonds with water molecules through its proton-accepting side chains, thus shifting the lowest unoccupied molecular orbital (LUMO) energy of water from −0.37 to −0.14 eV and inducing a negative shift in the onset potential for hydrogen evolution by 110 mV. Additionally, it immobilizes the counteranions onto the polymer backbones via covalent bonding, hence making the Zn2+ transference number nearly unity (0.96). Meanwhile, the high modulus PEM establishes a solid-state diffusion barrier to homogenize the interfacial Zn2+ flux, leading to 3D in-plane interfacial Zn2+ diffusion and compact Zn0 plating within the (002) plane. Atomic resolution scanning transmission electron microscopy (STEM) reveals corrosion-free Zn0 deposition without electrolyte degradation, while operando transition X-ray microscopy (TXM) further illustrates the real-time dendrite-free Zn0 plating process at 5 mA/cm2. Consequently, the unique properties of this water-binding and anion-tethering PEM enable enhanced electrochemical performance without employing highly fluorinated and expensive anions. This PEM demonstrates a durability of 3800 h in Zn0–Zn0 symmetric cells and a lifetime of 6000 cycles in Zn0–LiV3O8 full cells.
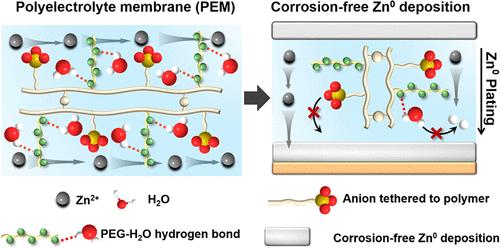
聚电解质膜通过固定阴离子和稳定水实现高度可逆的锌电池化学反应
由于水的电压窗口有限、移动反离子的界面副反应以及充电过程中锌金属枝晶的生长,水基电解质集成到锌离子电池中遇到了挑战。在本研究中,我们介绍了一种非氟化、阳离子导电的聚电解质膜(PEM),旨在通过抑制水和反离子的反应性来缓解这些挑战。该PEM通过其质子接受侧链与水分子形成氢键,从而将水的最低未占据分子轨道(LUMO)能量从- 0.37 eV转移到- 0.14 eV,并诱导氢演化的起始电位负移110 mV。此外,它通过共价键将反阴离子固定在聚合物骨架上,从而使Zn2+的转移数接近统一(0.96)。同时,高模量PEM建立了一个固态扩散屏障,使界面Zn2+通量均匀化,导致三维平面内界面Zn2+扩散,并在(002)平面内致密镀Zn0。原子分辨率扫描透射电子显微镜(STEM)显示无电解质降解的无腐蚀Zn0沉积,而operando转变x射线显微镜(TXM)进一步说明了5 mA/cm2的实时无枝晶Zn0镀过程。因此,这种水结合和阴离子束缚的PEM的独特性质使得无需使用高氟化和昂贵的阴离子即可增强电化学性能。该PEM在Zn0-Zn0对称电池中耐久性为3800小时,在Zn0-LiV3O8全电池中寿命为6000次。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


