Arylsulfonothioates: Thiol-Activated Donors of Hydropersulfides which are Excreted to Maintain Cellular Redox Homeostasis or Retained to Counter Oxidative Stress
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
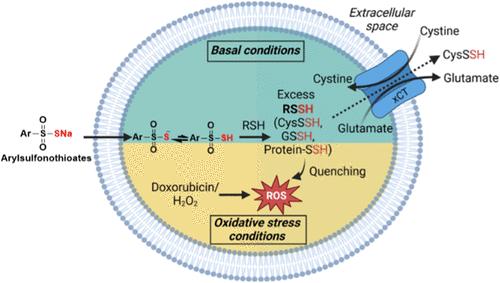
Despite their biological significance, the study of hydropersulfides (RSSH) is often limited due to their inherent instability. Here, we introduce arylsulfonothioates as thiol-activated RSSH donors and provide insight into cellular reactive sulfur species homeostasis. These precursors persulfidate physiologically relevant thiols (RSH) to form the corresponding RSSH. Real-time monitoring of hydrogen sulfide (H2S) generation via membrane inlet mass spectrometry (MIMS) was employed to follow RSSH production, revealing that electron-donating aryl substituents marginally slow RSSH release rates, whereas electron-withdrawing substituents slightly accelerate release. Furthermore, arylsulfonothioates with strong electron-withdrawing substituents offer superior protection against doxorubicin (DOX)-induced cardiotoxicity. Experiments using H9c2 cardiomyocytes affirmed the cell-permeability of arylsulfonothioates and their ability to increase intracellular RSSH levels and protein persulfidation levels. Notably, we observe the excretion of RSSH into the extracellular medium. Further investigations revealed the involvement of the cystine/glutamate antiporter SLC7A11, as cotreatment with its inhibitor, sulfasalazine, significantly reduce extracellular RSSH release. H9c2 cells exhibit tolerance to arylsulfonothioate 1g with an electron-withdrawing 4-cyano group at 1 mM; however, inhibition of the cystine antiporter results in a minor decrease in cell viability. Under oxidative stress conditions induced by DOX or hydrogen peroxide (H2O2), cotreatment with 1g diminishes the excretion of RSSH and confers cytoprotection against DOX or H2O2-mediated toxicity. Our findings show adaptive cellular responses to RSSH levels, demonstrating excretion under elevated conditions to maintain redox homeostasis and intracellular retention as a protective response during oxidative stress.
芳基磺硫酸盐:巯基活化的氢过硫化物供体,通过排泄来维持细胞氧化还原稳态或保留来对抗氧化应激
尽管氢过硫化物(RSSH)具有重要的生物学意义,但由于其固有的不稳定性,研究往往受到限制。在这里,我们介绍了芳基磺硫酸盐作为硫醇激活的RSSH供体,并提供了细胞活性硫物种稳态的见解。这些前体过硫化生理相关的硫醇(RSH)形成相应的RSSH。通过膜入口质谱(MIMS)实时监测硫化氢(H2S)的生成,跟踪RSSH的产生,发现提供电子的芳基取代基略微减缓了RSSH的释放速度,而吸电子取代基略微加速了释放速度。此外,具有强吸电子取代基的芳基磺硫代酸盐对阿霉素(DOX)诱导的心脏毒性具有良好的保护作用。利用H9c2心肌细胞进行的实验证实了芳基磺硫酸盐的细胞渗透性,以及它们增加细胞内RSSH水平和蛋白质过硫化水平的能力。值得注意的是,我们观察到RSSH排泄到细胞外培养基中。进一步的研究表明,胱氨酸/谷氨酸反转运蛋白SLC7A11与其抑制剂磺胺氮嗪共处理可显著减少细胞外RSSH释放。H9c2细胞在1 mM处对1g具有吸电子4-氰基的芳基磺硫代酸盐表现出耐受性;然而,抑制胱氨酸反转运蛋白会导致细胞活力的轻微下降。在DOX或过氧化氢(H2O2)诱导的氧化应激条件下,与1g共处理可减少RSSH的排泄,并对DOX或H2O2介导的毒性具有细胞保护作用。我们的研究结果显示了细胞对RSSH水平的适应性反应,表明在氧化应激时,在高条件下排泄以维持氧化还原稳态和细胞内保留作为一种保护性反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


