Engineering Helical Chirality in Metal-Coordinated Cyclodextrin Nanochannels
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
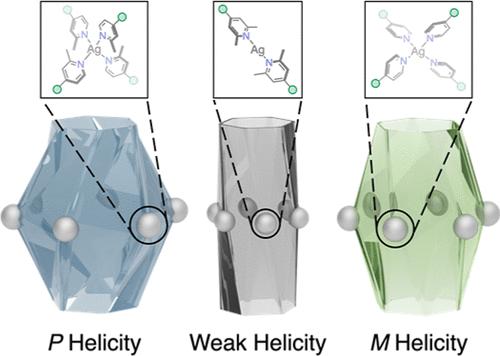
Helicates are a defining element of DNAs and proteins, with functions that are critical to a variety of biological processes. Cyclodextrins are promising candidates for forging multiple-stranded helicates with well-defined helicity, but a lack of available tools has precluded the construction of artificial helical nanochannels with a controllable geometry and helicity from these widely available chiral building blocks. Herein, we disclose a family of Ag6L2 helical nanochannels that can be readily assembled from α-cyclodextrin-derived ligands through coordination between pyridinyl groups and Ag+ cations. We discovered that the nanochannels exhibit either an M or a P helicity when the Ag+ cations adopt a tetrahedral coordination geometry while losing most of their helicity when the Ag+ cations are linearly coordinated. Both the geometry and helicity of the nanochannels can be precisely controlled by simply changing the number of methyl groups at the ortho positions of the pyridinyl ligands. The tetracoordinated Ag+ cations interconnect the helical nanochannels into an infinite two-dimensional coordinative network characterized by hexagonal tessellation. Theoretical calculations, which reveal lower energies of the helical conformations observed in crystals compared with those of their inverted counterparts, support the experimental results.
金属配位环糊精纳米通道的工程螺旋手性
螺旋是dna和蛋白质的定义元素,其功能对各种生物过程至关重要。环糊精是锻造具有明确螺旋度的多链螺旋的有希望的候选材料,但缺乏可用的工具,阻碍了利用这些广泛可用的手性构建块构建具有可控几何形状和螺旋度的人工螺旋纳米通道。在此,我们揭示了一个家族的Ag6L2螺旋纳米通道,可以很容易地通过吡啶基和Ag+阳离子之间的配位,由α-环糊精衍生的配体组装。我们发现,当银离子采用四面体配位几何时,纳米通道表现出M或P螺旋度,而当银离子采用线性配位时,纳米通道失去了大部分螺旋度。通过改变吡啶基配体邻位甲基的数量,可以精确地控制纳米通道的几何形状和螺旋度。四配位银离子将螺旋纳米通道互连成一个具有六边形镶嵌特征的无限二维配位网络。理论计算表明,在晶体中观察到的螺旋构象的能量比它们的反向构象低,这支持了实验结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


