Structure and Zeta Potential of Gold Nanoparticles with Coronas of Varying Size and Composition
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
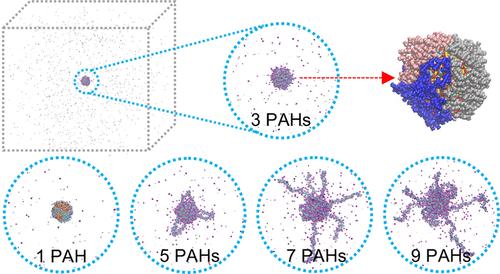
The structure of the soft ligand shell in engineered nanoparticles is related to their physical and chemical properties. The variation in that structre is critical for extending the diversity of functions in a wide variety of applications. To uncover the structure of soft PAH coronas wrapped on gold nanoparticles (AuNPs), in particular, we used atomistic simulations in this work. We found that increasing the number of PAH chains can increase both the size of the soft PAH corona and the magnitude of the electric potential of the PAH-wrapped cit-AuNPs (PAH-AuNPs). We also found that when the salt concentration increases, both the soft corona size and the electric potential decrease due to Debye screening. We compared the ligand structures, ion distributions, and electric potentials of 5 different nanoparticles─viz. citrate, PAH, 3-mercapto-propionic acid (MPA), 16-mercapto-hexadecyl-trimethylammonium bromide (MTAB), and hexadecyl-trimethylammonium bromide (CTAB) capped AuNPs. We found that when the surface charge densities are similar, these 5 different nanoparticles have similar electric potential profiles, but their ligand structures differ. Using Debye–Hückel theory, we determine the slipping planes (at the hydrodynamic radius, RH) and calculate the ζ-potentials of different AuNPs. We compared several machine learning (ML) models to predict the ζ-potential values learned from our simulation data and found that the Extra Trees model is the best at rationalizing the experimental data.
具有不同尺寸和组成的冠层的金纳米粒子的结构和 Zeta 电位
工程纳米颗粒的软配体壳结构与其物理和化学性质有关。该结构的变化对于在各种各样的应用中扩展功能的多样性至关重要。为了揭示包裹在金纳米粒子(AuNPs)上的软多环芳烃冕的结构,我们在这项工作中使用了原子模拟。我们发现,增加多环芳烃链的数量可以增加软多环芳烃电晕的大小和多环芳烃包裹的城市- aunps (PAH- aunps)的电位大小。我们还发现,当盐浓度增加时,由于Debye筛选,软电晕尺寸和电势都减小。我们比较了5种不同纳米粒子的配体结构、离子分布和电势。柠檬酸盐、多环芳烃、3-巯基-丙酸(MPA)、16-巯基-十六烷基-三甲基溴化铵(MTAB)和十六烷基-三甲基溴化铵(CTAB)覆盖AuNPs。我们发现,当表面电荷密度相似时,这5种不同的纳米粒子具有相似的电位分布,但它们的配体结构不同。利用debye - h ckel理论,我们确定了滑移面(流体动力半径,RH),并计算了不同aunp的ζ-势。我们比较了几种机器学习(ML)模型来预测从模拟数据中学习到的ζ-势值,发现Extra Trees模型在使实验数据合理化方面是最好的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


