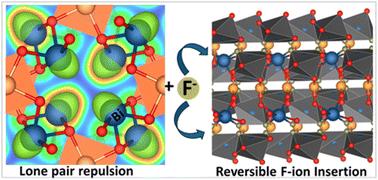
Stereochemical expression of Bi 6s2 lone pairs mediates fluoride-ion (De)insertion in tunnel-structured Bi2PdO4 and Bi1.6Pb0.4PtO4
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Fluoride-ion batteries are a promising alternative to lithium-ion batteries by dint of the greater crustal abundance of fluorine and the potential to alleviate the need for metal electrodeposition. However, conventional metal fluoride cathodes typically rely on conversion-type reactions that require propagation of a reaction—diffusion front, thereby limiting cycling performance and rate capability. In contrast, the topochemical insertion of fluoride-ions in periodic solids remains a relatively unexplored approach. Here, we explore the mechanisms of fluoridation of Bi2PdO4 and Bi1.6Pb0.4PtO4 insertion hosts that possess capacious tunnels that can accommodate fluoride-ions with a particular emphasis on elucidating the role of stereochemical expression of bismuth 6s2 lone pairs in mediating anion diffusion. We reveal that the topochemical solution-phase insertion and deinsertion of fluoride-ions at room temperature is mediated by redox reactions at platinum and palladium centers but involves multi-center synergies between d- and p-block atoms across the one-dimensional (1D) tunnel structure. While Pt and Pd centers mediate redox reactions, the stereochemically active lone pair electrons of Bi3+ play a pivotal role in facilitating reversible fluoride-ion diffusion. Consequently, Bi1.6Pb0.4PtO4 and Bi2PdO4 can be reversibly fluoridated with full recovery of the crystal lattice and with minimal alteration of the unit cell volume. The results reveal a key principle that the stereochemical activity of p-block electron lone pairs can be harnessed to modulate anion–lattice interactions and mediate facile anion diffusion.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


