Engineering the Lewis Acidity of Fe Single-Atom Sites via Atomic-Level Tuning of Spatial Coordination Configuration for Enhanced Oxygen Reduction
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nitrogen-doped carbon-supported Fe catalysts (Fe-N-C) with Fe-N4 active sites hold great promise for the oxygen reduction reaction (ORR). However, fine-tuning the structure of Fe-N4 active sites to enhance their performance remains a grand challenge. Herein, we report an innovative design strategy to promote the ORR activity and kinetics of Fe-N4 sites by engineering their Lewis acidity, which is achieved by tuning the spatial Fe coordination geometry. Theoretical calculations indicated that Fe1-N4SO2 sites (with an axial –SO2 group bonded to Fe) offered favorable Lewis acidity for the ORR, leading to optimized adsorption energies for the key ORR intermediates. To implement this strategy, we developed a molecular-cage-encapsulated coordination strategy to synthesize a Fe single-atom site catalyst (SAC) with Fe1-N4SO2 sites. In agreement with theory, the Fe1-N4SO2/NC catalyst demonstrated outstanding ORR performance in both alkaline (E1/2 = 0.910 V in 0.1 M KOH) and acidic media (E1/2 = 0.772 V in 0.1 M HClO4), surpassing commercial Pt/C and traditional Fe SACs with Fe1-N4 sites or planar S-coordinated Fe1-N4-S sites. Moreover, this newly developed catalyst showed great application potential in quasi-solid-state Zn–air batteries, delivering superior performance across a wide temperature range.
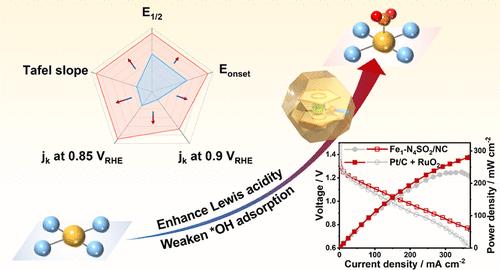
通过空间配位构型的原子级调整来增强氧还原,工程铁单原子位的刘易斯酸度
具有Fe- n4活性位点的氮掺杂碳负载铁催化剂(Fe- n- c)在氧还原反应(ORR)中具有广阔的应用前景。然而,微调Fe-N4活性位点的结构以提高其性能仍然是一个巨大的挑战。在此,我们报告了一种创新的设计策略,通过调整空间铁配位几何来调节Fe- n4位点的刘易斯酸度,从而提高其ORR活性和动力学。理论计算表明,Fe1-N4SO2位点(轴向-SO2基团与Fe键合)为ORR提供了有利的Lewis酸度,从而优化了ORR关键中间体的吸附能。为了实现这一策略,我们开发了一种分子笼封装配位策略来合成具有Fe1-N4SO2位点的Fe单原子位点催化剂(SAC)。与理论一致,Fe1-N4SO2/NC催化剂在碱性介质(0.1 M KOH中E1/2 = 0.910 V)和酸性介质(0.1 M HClO4中E1/2 = 0.772 V)中均表现出优异的ORR性能,优于具有Fe1-N4位或平面s配位Fe1-N4- s位的商用Pt/C和传统Fe SACs。此外,这种新开发的催化剂在准固态锌空气电池中显示出巨大的应用潜力,在宽温度范围内提供卓越的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


