Chemical Synthesis Reveals Pathogenic Role of N-Glycosylation in Microtubule-Associated Protein Tau
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
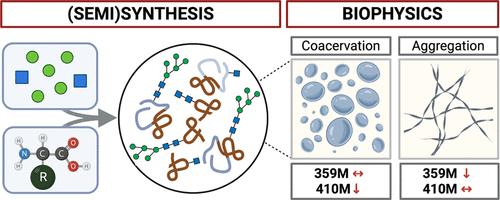
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by the accumulation of tau protein aggregates. In this study, we investigated the effects of N-glycosylation on tau, focusing on its impact on aggregation and phase behavior. We chemically prepared homogeneous glycoproteins with high-mannose glycans or a single N-acetylglucosamine at the confirmed glycosylation sites in K18 and 2N4R tau. Our findings reveal that N-glycosylation significantly alters biophysical properties and potentially cellular functions of tau. Small glycans promote tau aggregation and liquid–liquid phase separation (LLPS), while larger glycans reduce these effects. High mannose glycans at N410 enhance phosphorylation by GSK3β, suggesting a pathological role in AD. Functional assays demonstrate that N-glycosylation does not impact microtubule polymerization dynamics but modulates aggregation kinetics and morphology. This research underscores the importance of glycosylation in tau pathology and opens new avenues for therapeutic interventions targeting glycan processing.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


