Cyclic (Alkenyl)(Amino)Carbene (SMeCAenAC): Introducing a Member to the Cyclic (Alkyl)(Amino)Carbenes Family Featuring a Narrow Energy Gap
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
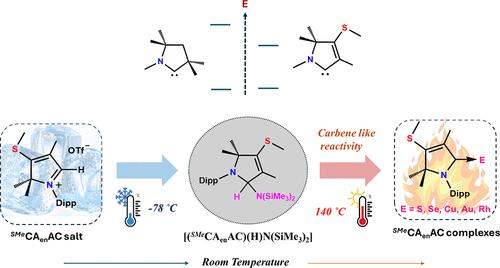
Herein, we report the carbene-like activity of a nonisolable, highly ambiphilic cyclic (alkenyl)(amino)carbene (SMeCAenAC, 3), which is stabilized as [(SMeCAenAC)(H)N(SiMe3)2] (4). This protected form (4) is stable in air and moisture. Compound 4 can be used as a carbene source through deamination upon heating to 130–140 °C. Moreover, density functional theory (DFT) calculations indicate that SMeCAenAC has the smallest singlet–triplet gap (37.05 kcal/mol) and a narrow highest occupied molecule orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) gap (3.92 eV) among the cyclic (alkyl)(amino)carbenes (CAACs). The precursor of carbene (3) can be synthesized on a multigram scale with a good yield. Moreover, the SMeCAenAC-coordinated copper complex showed excellent efficiency in the catalytic addition of phenols to electron-deficient olefins. This study also highlights that [SMeCAenAC-H]OTf can be used for metal-free catalysis, a property uniquely characteristic of an ambiphilic carbene, even though the formation of free SMeCAenAC (3) was not achieved.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


