Recombination of Autodissociated Water Ions in a Nanoscale Pure Water Droplet
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
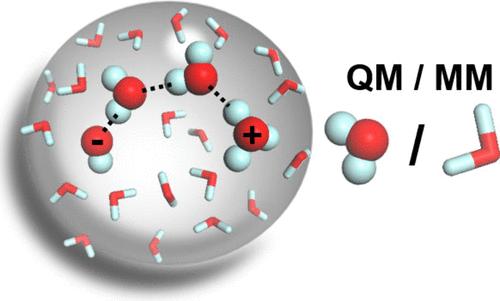
The recombination of water ions has diverse scientific and practical implications, ranging from acid–base chemistry and biological systems to planetary environments and applications in fuel cell and carbon conversion technologies. While spatial confinement affects the physicochemical properties of water dynamics, its impact on the recombination process has rarely been studied. In this work, we investigate the dynamics of water, the water ion distribution, and the ion recombination process in water droplets as a function of droplet size through molecular dynamics simulations and adaptive quantum mechanical/molecular mechanical calculations. We compare the dynamics of recombination in water droplet sizes ranging from 100 to 18 000 waters, both in their interiors and on their surfaces. We found that the self-diffusion of water dramatically decreases in droplets with a diameter below 2.2 nm. Using a classical RexPoN force-field, we found that the ions in 1000 H2O’s spend almost 50% of the time on the surface and 0.5 nm beneath it with a slight preference for OH– ion to reside longer on the surface. We estimate that, on average, recombination in these drops occurs at 400 ps in 1000 H2O’s and 1 ns in 3000 H2O’s. We also found that recombination is not limited by the local structure of the surface or the size of the droplet but can be influenced by the geometry of the water wire connecting the ions as they approach each other, which can often prevent recombination. Our results provide insights to the reaction microenvironments presented by nanoscopic water droplets.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


