Enantioselective modular synthesis of α-aryl-α-heteroaryl aminonitriles with parts per million organocatalyst loading: mechanistic investigation for stereochemical origins
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
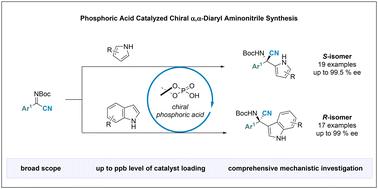
Heteroaromatic installation and peripheral modifications are the most common reactions in the pharmaceutical industry. However, the synthesis of biologically important aminonitrile-functionalized heteroaromatics remains unexplored. Although nucleophilic aminonitrile introduction and Strecker reaction under enantioselective catalytic conditions enable facile access to chiral aminonitriles, these approaches largely disfavor substrates with highly steric substituents on the imine carbon atom, thus affording limited products. Herein, we report an efficient and versatile method that combines the traditional methods to generate α-aryl-α-heteroaryl-aminonitriles. This methodology exhibits a broad scope and can form bonds even when using low-reactive Friedel–Crafts nucleophiles through a mild and practical protocol. It should be highlighted that the catalyst loading could be reduced to parts per billion, giving rise to phenomenal turn-over-number (TON) and turn-over-frequency (TOF) values. Interestingly, different stereochemistries between the pyrrole and indole adducts were obtained with the same (R)-derived chiral phosphoric acid catalysis. Computational studies have indicated that this unpredicted stereoreversal is due to the coordination system between iminonitriles and catalysts, helping us understand the origin of the stereochemical outcome of the traditional Friedel–Crafts reaction.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


