Robust Heteronuclear Correlations for Sub-milligram Protein in Ultrafast Magic-Angle Spinning Solid-State NMR
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
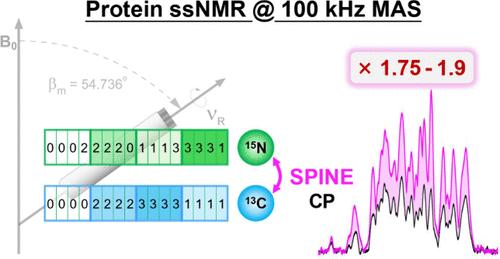
Proton-detected solid-state nuclear magnetic resonance (ssNMR) under ultrafast magic-angle spinning (MAS) has become a powerful tool for elucidating the structures of proteins with sub-milligram quantities, where establishing 13C–15N correlations is essential. However, traditional 13C–15N cross-polarization (CP), effective at lower MAS frequencies, suffers diminished efficiency under ultrafast MAS conditions. To overcome this limitation, we developed a robust method for selective polarization between insensitive nuclei (SPINE). This approach significantly enhances the heteronuclear 13C–15N correlation efficiency over CP, with gain factors of 1.75 for 13CA–15N and 1.9 and 13CO–15N transfers. SPINE’s efficacy was validated on four diverse proteins: the microcrystalline β1 immunoglobulin binding domain of protein G (GB1), the large-conductance mechanosensitive ion channel from Methanosarcina acetivorans (MaMscL), fibrillar septum-forming protein (SepF), and the vertex protein of the β-carboxysome shell (CcmL). This enhancement can reduce the duration of current multidimensional experiments to about one-third of that using a single 13C–15N CP and to about one-tenth with dual 13C–15N transfers. Our findings underscore the practical utility and versatility of SPINE in ssNMR spectroscopy, making it a valuable approach for advancing structural biology studies of sub-milligram protein.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


