Palladium-Catalyzed Asymmetric Acetoxylative Cyclization/Acyl Transfer Cascade of Alkyne-Tethered Malononitriles with Carboxylic Acids
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Palladium-catalyzed asymmetric intermolecular trans-acetoxypalladation/desymmetric cyclization/acyl transfer cascades of alkyne-tethered malononitriles with carboxylic acids have been demonstrated. Such a sequence enables the formation of multifunctionalized nitriles bearing α-all-carbon quaternary stereocenters with a high degree of enantiocontrol with a broad substrate scope. Moreover, synthetic elaborations present these multifunctionalized molecules as promising chiral building blocks. Mechanistic studies illustrate that the cascade process proceeds via a key imine intermediate, and the desymmetric cyclization is the enantio-determining step.
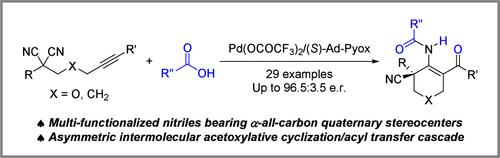
钯催化丙二腈与羧酸的不对称乙酰化环化/酰基转移级联反应
钯催化的炔系丙二腈与羧酸的不对称分子间反乙酰化/不对称环化/酰基转移级联反应已被证实。这样的序列使得形成具有α-全碳四元立体中心的多官能化腈,对映体控制程度高,底物范围广。此外,合成精化将这些多功能化分子作为有前途的手性构建块。机制研究表明,级联过程通过一个关键的亚胺中间体进行,不对称环化是对映体决定步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


