Half-Sandwich Ru(II) Complexes Featuring Metal-Centered Chirality: Configurational Stabilization by Ligand Design, Preparation via Kinetic Resolution, and Application in Asymmetric Catalysis
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
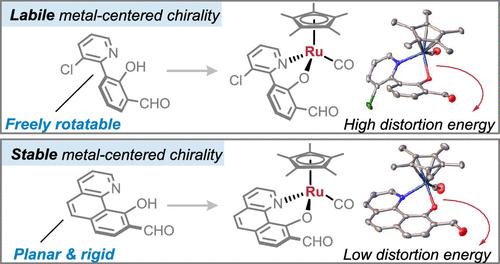
While it is well established that half-sandwich Ru(II) complexes possess metal-centered chirality, effective strategies to leverage their metal-centered chirality toward asymmetric synthesis have been challenging due to the configurational lability of the metal stereocenters. The metal-centered chirality is typically mediated by enantiopure ligands, which occupy a relatively narrow chemical space compared to their achiral counterparts. We demonstrate that achiral ligands can be used to access chiral-at-ruthenium half-sandwich complexes with exceptionally high configurational stability. Key to success is the introduction of a rigid bidentate ligand with a minimized dihedral angle between the pyridyl and phenolic moieties, which proved effective for preventing racemization. Computational studies revealed the energetic factors contributing to the exceptional configurational stability enabled by the rigid and planar structure of the successful ligand design. These optically active chiral-at-ruthenium complexes incorporating aldehyde moieties were obtained by NHC-catalyzed kinetic resolution with excellent selectivities (s-factor up to >200, ee up to 99%). We further demonstrate that they are highly effective chiral aldehyde catalysts for the asymmetric 1,6-conjugate addition of glycine ester and para-quinone methide.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


