Stability of RbO2 as a Discharge Product of Metal–O2 Batteries
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
This study presents evidence that the discharge products of positive electrodes in metal–oxygen batteries using Rb- and Cs-based electrolytes are RbO2 and CsO2, paralleling the findings for Na- and K-based systems. We explored the positive electrode reaction inside alkali metal–oxygen batteries, revealing that the stability of MO2 compounds (M = Na, K, Rb, Cs) is influenced by the alkali metal ion sizes, achieving maximum reversibility with Rb+ ions. Despite the high sensitivity of metal–oxygen batteries to trace amounts of moisture and CO2 within the cell, we successfully demonstrated stable charge–discharge cycles with a nonaqueous Rb-based electrolyte.
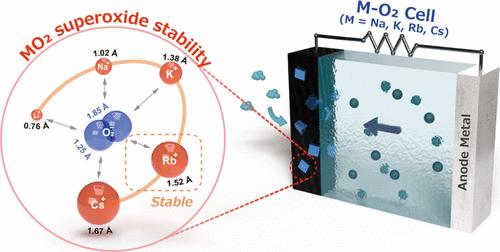
金属- o2电池放电产物RbO2的稳定性
本研究提供证据表明,在金属氧电池中,使用Rb基和cs基电解质的正极放电产物是RbO2和CsO2,与Na基和Na基系统的发现相似。我们探索了碱金属-氧电池内部的正极反应,揭示了MO2化合物(M = Na, K, Rb, Cs)的稳定性受到碱金属离子大小的影响,Rb+离子的可逆性最大。尽管金属氧电池对电池内微量水分和二氧化碳的敏感性很高,但我们成功地证明了使用非水铷基电解质的稳定充放电循环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


