Synthesis of Cationic Cyclic Oligo(disulfide)s via Cyclo-Depolymerization: A Redox-Responsive and Potent Antibacterial Reagent
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Antimicrobial peptides (AMPs) and synthetic topologically defined peptide mimics have been developed as alternatives to traditional small-molecule antibiotics. AMP mimetics arising from linear polymers used widely in preclinical studies have shown promise but have limited stability. Oligomers possessing cyclic topology have been proposed to have increased stability but remain understudied due to synthetic challenges and concerns over cytotoxicity. Herein, we present an efficient approach to prepare cationic, cyclic oligo(disulfide)s (CCOs) from lipoic acid derivatives. The CCOs are obtained in a one-pot cascade reaction of ring-opening polymerization preceding an in situ cyclo-depolymerization. CCOs are effective against a broad spectrum of bacteria, exhibiting a 5.43-log reduction in 5 min against Escherichia coli. They did not induce antimicrobial resistance during 24 successive passages in vitro. The cytotoxicity of CCOs is reduced by exploiting glutathione-triggered degradation. Further, fine-tuning of the cationic-to-hydrophilic ratio in CCOs has yielded improved stability in serum and a high selective index (HC50/MIC > 1280) against methicillin-resistant Staphylococcus aureus. In an infected wound rodent model, CCOs have shown substantial antibacterial potency against S. aureus, underscoring their therapeutic potential as a new class of antimicrobial agents.
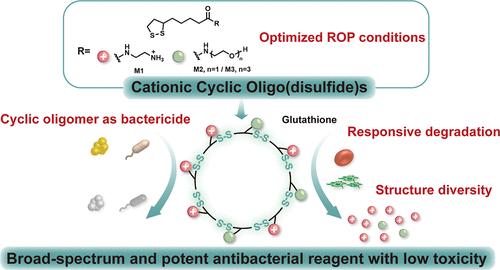
环解聚法合成阳离子环低聚二硫:一种具有氧化还原反应的高效抗菌试剂
抗菌肽(AMPs)和合成的拓扑定义肽模拟物已经发展成为传统小分子抗生素的替代品。在临床前研究中广泛使用的线性聚合物产生的AMP模拟物已经显示出希望,但稳定性有限。具有环状拓扑结构的低聚物已被提出具有更高的稳定性,但由于合成挑战和对细胞毒性的担忧,仍未得到充分研究。本文提出了一种从硫辛酸衍生物中制备阳离子环低聚二硫醚(CCOs)的有效方法。CCOs是在开环聚合的一锅级联反应中获得的,然后进行原位环解聚。CCOs对广泛的细菌有效,对大肠杆菌5分钟内降低5.43对数。在体外连续24次传代均未诱导抗微生物药物耐药性。利用谷胱甘肽引发的降解可以降低CCOs的细胞毒性。此外,CCOs中阳离子与亲水性比例的微调提高了血清稳定性和高选择性指数(HC50/MIC >;1280)抗耐甲氧西林金黄色葡萄球菌。在感染伤口的啮齿动物模型中,CCOs对金黄色葡萄球菌显示出显著的抗菌效力,强调了其作为一类新型抗菌药物的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


