High-Temperature Growth of CeOx on Au(111) and Behavior under Reducing and Oxidizing Conditions
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
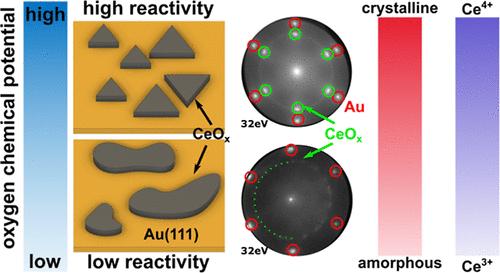
Inverse oxide–metal model catalysts can show superior activity and selectivity compared with the traditional supported metal–oxide architecture, commonly attributed to the synergistic overlayer–support interaction. We have investigated the growth and redox properties of ceria nanoislands grown on Au(111) between 700 and 890 °C, which yields the CeO2–Au(111) model catalyst system. We have observed a distinct correlation between deposition temperature, structural order, and oxide composition through low-energy electron microscopy, low-energy electron diffraction, intensity–voltage curves, and X-ray absorption spectroscopy. Improved structural order and thermal stability of the oxide have been achieved by increasing the oxygen chemical potential at the substrate surface using reactive oxygen (O/O2) instead of molecular O2 during growth. In situ characterization under reducing (H2) and oxidizing atmospheres (O2, CO2) indicates an irreversible loss of structural order and redox activity at high reduction temperatures, while moderate temperatures result in partial decomposition of the ceria nanoislands (Ce3+/Ce4+) to metallic cerium (Ce0). The weak interaction between Au(111) and CeOx would facilitate its reduction to the Ce0 metallic state, especially considering the comparatively strong interaction between Ce0 and Au0. Besides, the higher reactivity of atomic oxygen promotes a stronger interaction between the gold and oxide islands during the nucleation process, explaining the improved stability. Thus, we propose that by driving the nucleation and growth of the ceria/Au system in a highly oxidizing regime, novel chemical properties can be obtained.
氧化铈在Au(111)上的高温生长及其在还原氧化条件下的行为
与传统的负载型金属-氧化物结构相比,逆氧化物-金属模型催化剂表现出更好的活性和选择性,这通常归因于协同的覆盖层-载体相互作用。我们研究了在700 ~ 890°C之间在Au(111)上生长的氧化铈纳米岛的生长和氧化还原性能,得到了CeO2-Au(111)模型催化剂体系。我们通过低能电子显微镜、低能电子衍射、强度-电压曲线和x射线吸收光谱观察到沉积温度、结构顺序和氧化物组成之间存在明显的相关性。通过在生长过程中使用活性氧(O/O2)代替分子氧来增加衬底表面的氧化学势,可以改善氧化物的结构秩序和热稳定性。还原气氛(H2)和氧化气氛(O2, CO2)下的原位表征表明,在高还原温度下,结构秩序和氧化还原活性不可逆地丧失,而中等温度导致铈纳米岛(Ce3+/Ce4+)部分分解为金属铈(Ce0)。Au(111)与CeOx之间的弱相互作用有利于其还原为Ce0金属态,特别是考虑到Ce0与Au0之间相对较强的相互作用。此外,在成核过程中,原子氧的高反应活性促进了金岛和氧化岛之间更强的相互作用,这解释了稳定性的提高。因此,我们提出,通过在高氧化状态下驱动铈/Au体系的成核和生长,可以获得新的化学性质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


