Reduction-Interrupted Tandem Reaction for General Synthesis of Functional Amino Acids by a Heterogeneous Cobalt Catalyst
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Despite their significant importance in numerous fields, the challenges in direct and diverse synthesis of γ-amino-α-hydroxybutyric acids (AHBAs) pose substantial obstacles to explore their functions. Here, by preparation of a N-doped carbon-supported bifunctional cobalt catalyst (Co-DAPhen/C), it was applied to develop a reductive tandem reaction for general synthesis of AHBA derivatives from cheap and abundant nitroarenes, formaldehyde, and acrylates. This catalytic three-component reaction features broad substrate and functionality tolerance, an easily accessible and reusable catalyst, and high step and atom economy. The active Co sites of the catalyst are involved in the mild reduction processes with formic acid, whereas the N-doped carbon support enriches the HCHO and acrylates by physical adsorption, thus favoring the capture of hydroxylamine and nitrone intermediates via condensation and 1,3-dipolar cycloaddition, respectively. Such a metal–support synergy interrupts the conventional reduction of nitroarenes into anilines and results in a novel tandem reaction route. In this work, the concept merging mild reduction and effective intermediate transformations is anticipated to develop more useful tandem reactions by rational catalyst design.
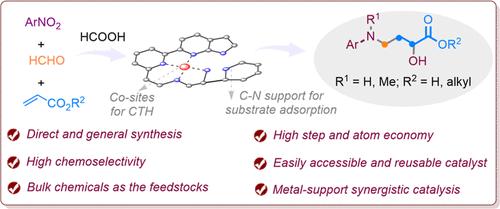
尽管γ-氨基-α-羟基丁酸(AHBAs)在众多领域具有重要意义,但其直接和多样化合成的挑战对探索其功能构成了巨大障碍。本文通过制备一种掺杂 N 的碳支撑双功能钴催化剂(Co-DAPhen/C),将其用于开发一种还原串联反应,以廉价而丰富的硝基烯烃、甲醛和丙烯酸酯为原料合成 AHBA 衍生物。这种催化三组分反应具有广泛的底物和官能度耐受性、催化剂易于获得和重复使用、高步长和原子经济性等特点。催化剂的活性 Co 位点参与了与甲酸的温和还原过程,而掺杂 N 的碳载体则通过物理吸附富集了 HCHO 和丙烯酸酯,从而有利于通过缩合和 1,3-二极环加成分别捕获羟胺和腈酮中间体。这种金属支撑协同作用中断了传统的将硝基烯烃还原成苯胺的过程,形成了一种新的串联反应路线。在这项工作中,将温和还原与有效中间转化相结合的概念有望通过合理的催化剂设计开发出更有用的串联反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


