Harnessing Oxidized Amines as Robust Sorbents for Carbon Capture
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
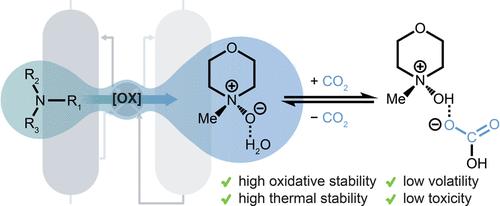
Carbon capture and sequestration (CCS) is imperative to mitigating global climate change, but current implementation falls far short of that needed to reach net-zero global emissions by 2050. Aqueous amine solutions, conceived over a century ago, are the current leading technology for CO2 separations. However, amines suffer from chemical instability under scrubbing conditions, corrosiveness, and toxicity, hindering their long-term implementation at multiton scales. Herein, we demonstrate for the first time that tertiary amine N-oxides, an oxidative degradation product of amines, can remove CO2 from dilute streams, including flue gas from a natural gas-fired power plant. Our extensive spectroscopic and computational studies support that the nontoxic, noncorrosive, and inexpensive 4-methylmorpholine N-oxide (MMNO) captures CO2 under humid conditions via the formation of a hydrogen-bond-stabilized bicarbonate (HCO3–) species, despite being significantly less basic than an amine. Accelerated aging studies show that MMNO exhibits superior oxidative and thermal stability compared to structurally similar amines, highlighting the potential of eco-friendly N-oxides in industrial carbon capture applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


