Enantioselective synthesis of isoxazolines bearing allenes by palladium-catalyzed carboetherification of β,γ-unsaturated ketoximes with propargylic acetates†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d4qo02319f
引用次数: 0
Abstract
A highly efficient Pd-catalyzed enantioselective carboetherification reaction of β,γ-unsaturated ketoximes with propargylic acetates is demonstrated. This method provides various chiral isoxazolines bearing multisubstituted allene groups in good yields with excellent enantioselectivities. The sterically electron-rich BINOL-derived phosphoramidite ligand exhibits highly efficient control over both chemoselectivity and enantioselectivity. The salient features of this reaction include readily available starting materials, broad functional group tolerance, an easy scale-up, versatile transformations and promising photo-physical properties. DFT calculations were carried out to disclose the detailed mechanism and origins of the enantioselectivity.
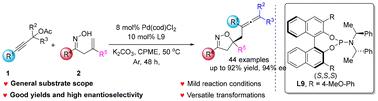
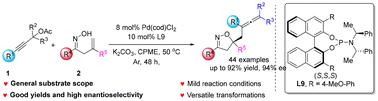
钯催化β,γ-不饱和酮肟与丙炔乙酸酯的碳醚化对映选择性合成含烯的异恶唑啉
研究了pd催化β,γ-不饱和酮肟与丙炔乙酸酯的高效对映选择性碳醚化反应。该方法制备了多种手性异恶唑类含多取代烯基的化合物,收率高,对映选择性好。立体富电子的binol衍生的磷酰胺配体在化学选择性和对映体选择性上都表现出高效的控制。该反应的显著特点包括:现成的起始材料、广泛的官能团耐受性、易于放大、多用途转化和有前途的光物理性质。DFT计算揭示了对映体选择性的详细机理和来源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


