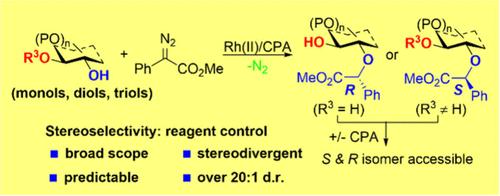
Rh(II) and Chiral Phosphoric Acid Co-catalyzed Selective O–H Insertions for Stereodivergent O-Alkylation of Glycosides
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Carbohydrates are synthetically challenging molecules with essential biological functions in all living systems. The selective synthesis and modification of carbohydrates are crucial for investigating their biological functions. Controlling chemo-, regio-, and stereoselectivity is a central theme in carbohydrate synthesis. Achieving the full set of stereoisomers of carbohydrate derivatives would significantly enhance the efficiency of building compound libraries for biological studies and drug discovery. However, the selective functionalization of seemingly identical hydroxyl groups in carbohydrates remains a long-standing challenge in organic chemistry. In carbohydrate synthesis, achieving precise control of both relative configurations in catalyst-controlled reactions that create a new stereocenter presents a significant synthetic challenge. Herein, we developed an efficient method for the stereodivergent O-alkylation of carbohydrate hydroxyl groups via Rh(II)/chiral phosphoric acid-cocatalyzed insertion of metal carbenoids. This system is mild and robust, offering excellent selectivity across a broad range of substrates with high regio- and stereoselectivity. Furthermore, this strategy opens up vast opportunities for stereodivergent synthesis.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


