Streamlining Sulfated Oligosaccharide and Glycan Synthesis with Engineered Mutant 6-SulfoGlcNAcases
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
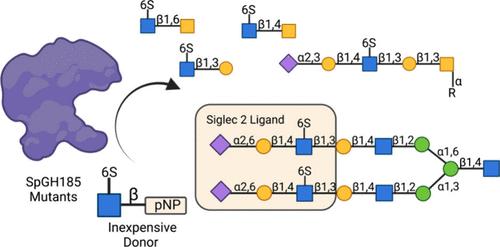
Sulfation is a common, but poorly understood, post-glycosylational modification (PGM) used to modulate biological function. To deepen our understanding of the roles of various sulfated glycoforms and their relevant binding proteins, we must expand our enzymatic toolkit for their synthesis. Here, we bypass the need for both sulfotransferases and glycosyltransferases by engineering a series of mutants of a 6-SulfoGlcNAcase, from Streptococcus pneumoniae, to directly and efficiently synthesize not only the ubiquitous 6S-GlcNAc-β-1,3-Gal linkage prevalent within host glycans, but also the 6S-GlcNAc-β-1,6-GalNAc commonly observed within core-6 O-glycans, and the more exotic 6S-GlcNAc-β-1,4-GalNAc linkage. We further elaborate these into complex sulfated N-glycan and O-glycan structures of biological relevance. By utilizing the cost-effective activated donor pNP-6S-GlcNAc in conjunction with mutant GH185 6-SulfoGlcNAcases we demonstrate a simple yet powerful in vitro method for generating well-defined sulfated oligosaccharides and glycoforms for use in a variety of applications including glycan arrays, glycan remodeling, and specificity studies with carbohydrate binding proteins such as lectins.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


