Genomic mediators of acquired resistance to immunotherapy in metastatic melanoma
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
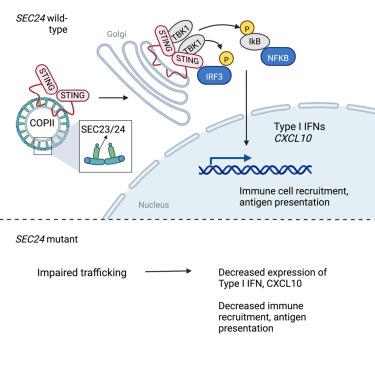
Although some patients with metastatic melanoma experience durable responses to immune checkpoint inhibitors (ICIs), most exhibit intrinsic or acquired resistance to these therapies. Here, we compare somatic genomic profiles from matched pre-treatment and post-resistance tumor biopsies in patients (n = 25) with metastatic melanoma who exhibited heterogeneous ICI responses to nominate additional mediators of acquired resistance. We find that several acquired resistance tumors exhibit defects in B2M or JAK1/2, consistent with prior findings. We also discover resistance-associated mutations in SEC24C and SEC24D in 3 patients. SEC24 has an essential role in the trafficking of the dsDNA sensor STING and has been linked to interferonopathies. Melanoma cells engineered to express the SEC24C mutations observed in patients exhibit diminished STING signaling, including decreased type I interferon production, antigen presentation, and a reduced capacity to activate cytotoxic T cells. This study nominates a role for aberrant STING trafficking in acquired resistance to ICIs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


