Dynamics of molecular heterogeneity in high-risk luminal breast cancer—From intrinsic to adaptive subtyping
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
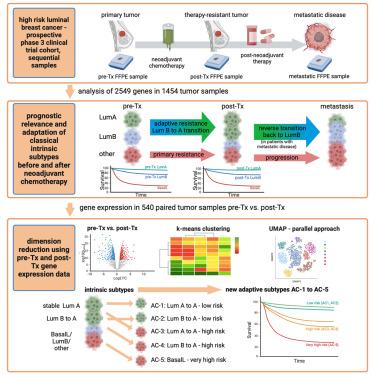
We evaluate therapy-induced molecular heterogeneity in longitudinal samples from high-risk, hormone-receptor positive/HER2-negative breast cancer patients with residual tumor after neoadjuvant chemotherapy from the Penelope-B trial (NCT01864746; EudraCT 2013-001040-62). Intrinsic subtypes are prognostic in pre-therapeutic (Tx) samples (n = 629, p < 0.0001) and post-Tx residual tumors (n = 782, p < 0.0001). After neoadjuvant chemotherapy, a shift of intrinsic subtypes is observed from pre-Tx luminal (Lum) B to post-Tx LumA, with reverse transition back to LumB in metastases. In a combined analysis of 540 paired pre-Tx and post-Tx samples, we identify five adaptive clusters (AC-1–5) based on transcriptomic changes before and after neoadjuvant chemotherapy. These AC-subtypes are prognostic beyond classical intrinsic subtyping, categorizing patients into groups with excellent prognosis (AC-1 and AC-2), poor prognosis (AC-3 and AC-4), and very poor prognosis (AC-5, enriched for basal-like subtype). Our analysis provides a basis for an extended molecular classification of breast cancer patients and improved identification of high-risk patient populations.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


