Multi-dimensional, Multi-scale Analysis of Interphase Chemistry for Enhanced Fast-Charging of Lithium-Ion Batteries with Ion Mass Spectrometry
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
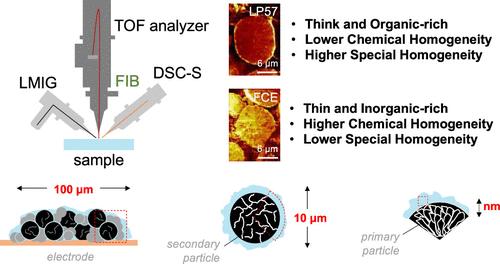
Understanding the fundamental properties of electrode–electrolyte interphases (EEIs) is essential for designing electrolytes that support stable operation under high charging rates. In this study, we benchmark our fast-charging electrolyte (FCE) against the commercial LP57 electrolyte to identify the EEI characteristics that enhance fast-charging performance. By utilizing the latest advances in time-of-flight secondary ion mass spectrometry (TOF-SIMS) and focused-ion beam (FIB) techniques, we reveal the complex chemical architecture of the cathode–electrolyte interphase (CEI). Our findings indicate that stable battery operation under fast-charging conditions requires reduced surface reactivity rather than stabilizing the bulk integrity of the cathode. While inorganic species are often cited as beneficial for EEI composition, their distribution within the EEI is equally critical. Additionally, dynamic interactions between the cathode material and conductive carbon significantly affect CEI formation and alter the passivation layer chemistry. A chemically homogeneous distribution of CEI components passivating preferentially the active material particles is desired for enhanced performance. Notably, the amount of electrolyte decomposition species in the solid-electrolyte interphase (SEI) far outweighs their distribution within the SEI in determining better electrochemical performance. An inorganic-rich SEI effectively protects graphite particles, suppresses the accumulation of metallic lithium, and prevents the formation of lithium dendrites. Overall, an enhanced fast-charging performance can be achieved by tuning the interphase chemistry and architecture on both the cathode and anode sides.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


