Ion-Specific Effects under Electric Fields on Ice Nucleation at the Mica Surface
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Applying external electric fields to mineral surfaces can have a substantial impact on ice nucleation, influencing both climate and atmospheric systems. While earlier studies have demonstrated that electric fields can enhance ice nucleation on nonmineral surfaces, the mechanisms driving heterogeneous ice nucleation (HIN) on mineral surfaces under electric fields with different surface ions remain unclear. In this study, we investigate the ion-specific effects under electric fields on HIN efficiency using mica surfaces containing various cations. Our findings reveal that an upward electric field significantly boosts HIN of water droplets atop Na-mica surfaces, raising the nucleation temperature by approximately 6 °C. In contrast, mica surfaces with other cations or those exposed to a downward electric field show no change in nucleation temperature or HIN efficiency. Molecular dynamics simulations suggest that Na+ ions detach more easily from the mica surface under an electric field, exposing more of the flat mica lattice and thus possibly promoting ice nucleation. This study offers new insights into the ion-specific effects of electric fields on HIN, providing a deeper understanding of the role of cations and electric fields in ice nucleation processes.
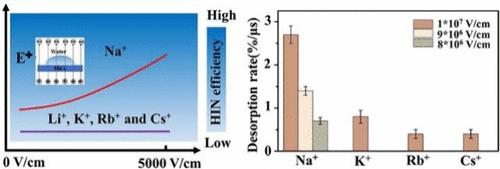
电场作用下离子对云母表面冰核的影响
在矿物表面施加外部电场可对冰成核产生重大影响,影响气候和大气系统。虽然早期的研究表明电场可以增强非矿物表面的冰核,但在不同表面离子的电场作用下,驱动矿物表面非均相冰核(HIN)的机制尚不清楚。在本研究中,我们利用含有不同阳离子的云母表面,研究了电场下离子特异性对HIN效率的影响。研究结果表明,向上电场显著提高了纳米云母表面水滴的HIN,使成核温度提高了约6℃。相比之下,含有其他阳离子的云母表面或暴露在向下电场下的云母表面的成核温度和HIN效率没有变化。分子动力学模拟表明,Na+离子在电场作用下更容易从云母表面分离,暴露出更多的平面云母晶格,从而可能促进冰核。本研究为电场对HIN的离子特异性效应提供了新的见解,为阳离子和电场在冰成核过程中的作用提供了更深入的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


