Ketenimines as Aza-Dienophiles
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
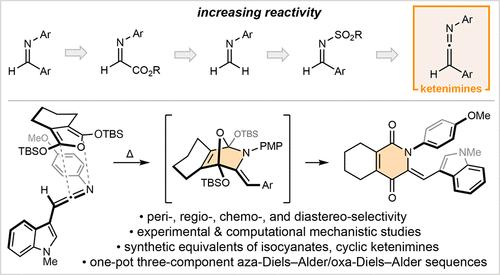
N-Aryl ketenimines have been established as highly reactive aza-dienophiles. Intermolecular cycloadditions are achieved upon heating in the presence of 2,5-bis(silyloxy)furans and proceed with high levels of peri-, regio-, chemo- and diastereo-selectivity. Spontaneous C–O cleavage yields oxygenated pyridone derivatives in a highly convergent and redox-neutral manner. Combined experimental and computational studies demonstrate N-aryl ketenimines to be significantly more reactive than imino dienophiles, as a consequence of less distorted transition states. Derivatization studies include the development of isocyanate and cyclic ketenimine equivalents as aza-dienophiles, while extension to a one-pot aza-Diels–Alder/oxa-Diels–Alder sequence provides a three-component approach to complex fused pyridone/pyran systems.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


