Hypoxia combined with radiation reverses migration and invasion of head and neck squamous cell carcinoma by remodeling extracellular vesicle-mediated transfer of miR-23b-5p from cancer-associated fibroblasts
Abstract
Background
Cancer-associated fibroblasts (CAFs), the main matrix components in the tumor microenvironment (TME), play a crucial role in tumor progression. Extracellular vesicles (EVs) as main mediators in intercellular communication can be regulated by hypoxia or radiation.
Methods
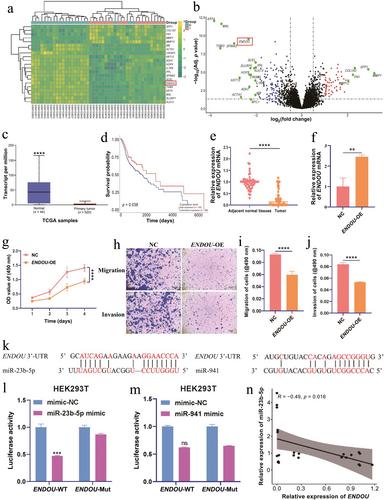
CAFs were extracted from head and neck squamous cell carcinoma (HNSCC) tissues and CAF-derived EVs were collected by ultracentrifugation. Bioinformatics analysis determined the role of poly (U)-specific endonuclease (ENDOU) on HNSCC progression and confirmed that ENDOU inhibited HNSCC progression by overexpressing ENDOU in HNSCC. Dual-luciferase activity report assay confirmed that miR-23b-5p was involved in the regulation of ENDOU expression. The migration and invasion of HNSCC cells were verified by transwell assay. Furthermore, tumor-bearing mouse models were used to demonstrate the potential of EVs loaded with miR-23b-5p in HNSCC to promote tumor progression.
Results
Our results showed that ENDOU was downregulated in HNSCC and inhibited HNSCC migration and invasion. Hypoxia and radiotherapy reversed CAF-derived EVs to promote migration and invasion of HNSCC. Mechanically, hypoxia and radiation downregulated miR-23b-5p in CAF-derived EVs and then restored ENDOU expression in HNSCC. Finally, CAF-derived EVs carrying miR-23b-5p promoted the progression of HNSCC cells in vivo by regulating ENDOU expression.
Conclusion
This study demonstrated that hypoxia combined with radiation reverses the promoting effect of CAFs on HNSCC migration and invasion by reducing the delivery of miR-23b-5p by CAF-derived EVs to decrease the inhibitory effect of ENDOU expression in HNSCC. The results provide a new perspective for better understanding the role of stromal components in TME in tumor regulation. Furthermore, the results provide a strong basis for the possibility of ENDOU as a biomarker for HNSCC.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


