Postpolymerization Modification of Poly(2-isopropenyl-2-oxazoline) with Thiols: Scope and Solvent Effects
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Poly(2-isopropenyl-2-oxazoline) (PIPOx) has emerged as a unique postpolymerization modification platform; however, only the relatively slow PIPOx reactions with carboxylic acids are currently routinely exploited. Here, we provide the first comprehensive investigation into PIPOx reactivity with different aliphatic and aromatic thiols. We revealed that these reactions are dramatically accelerated in water, affording high degrees of modification (DM) faster and/or under milder reaction conditions than in organic solvents. This was particularly important for the generally less-reactive aliphatic thiols, where we demonstrated high DM values for the first time. Aromatic thiols were found to be comparatively more reactive, with reactivity differences ascribed largely to the thiol acidity governed by substitution effects. Besides enhancing the reaction rate, the aqueous medium also greatly simplified product isolation in many cases. Furthermore, a precise stoichiometric control over DM, enabling precision tailoring of (co)polymer composition, was manifested for model thiols. Finally, we exemplified how thiol reactions can be used for the rapid and clean incorporation of a range of useful functionalities into the parent polymer, imparting important functional properties or creating reactive sites for further polymer modifications, including cross-coupling and para-fluoro-thiol click reactions. Our findings extend significantly the PIPOx-based postpolymerization modification platform.
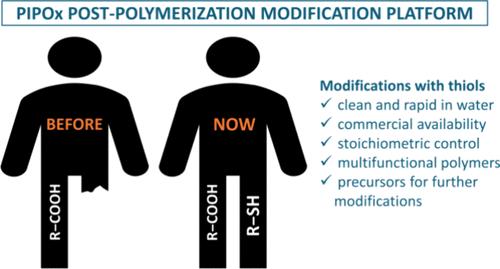
巯基聚(2-异丙烯-2-恶唑啉)的聚合后改性:范围和溶剂效应
聚(2-异丙烯-2-恶唑啉)(PIPOx)是一种独特的聚合后改性平台;然而,目前只有相对缓慢的PIPOx与羧酸的反应被常规利用。在这里,我们首次全面研究了PIPOx与不同脂肪族和芳香硫醇的反应性。我们发现,与有机溶剂相比,这些反应在水中显著加速,在更温和的反应条件下,可以更快地进行高度修饰(DM)。这对于通常反应性较低的脂肪族硫醇尤其重要,我们首次证明了高DM值。芳香族硫醇的反应性相对较强,其反应性差异主要归因于由取代效应控制的硫醇酸度。水介质除了提高反应速率外,在许多情况下也大大简化了产物的分离。此外,对模型硫醇进行了精确的DM化学计量控制,从而实现了(co)聚合物组成的精确裁剪。最后,我们举例说明了如何利用硫醇反应快速而干净地将一系列有用的功能结合到母体聚合物中,赋予重要的功能特性或为进一步的聚合物修饰创造反应位点,包括交叉偶联和对氟硫醇点击反应。我们的发现大大扩展了基于pipox的聚合后修饰平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


