Sialylation Shields Glycoproteins from Oxidative Stress: Mechanistic Insights into Sialic Acid Oxidation and Structural Stability
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
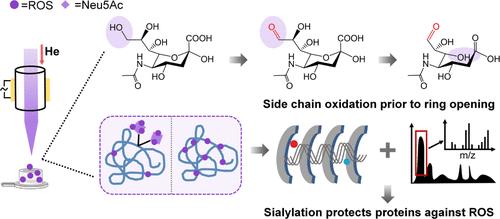
Sialylation, a crucial yet labile protein modification, is increasingly recognized for its role in modulating protein structure, function, and stability. While the impact of oxidative stress on protein integrity is well-established, the protective role of sialylation against such damage remains poorly understood. This study employs a microscale low-temperature plasma device to generate a controlled, deep radical oxidation environment mimicking cellular oxidative stress. By subjecting free sialic acids (Neu5Ac and Neu5Gc) to time-resolved deep radical exposure, high-resolution mass spectrometry, and high-fidelity density functional theory calculations, we establish an unprecedented oxidation pathway, revealing unique stepwise side chain oxidation prior to ring opening. Comprehensive radical oxidation maps comprising over 100 oxidative intermediates provide a molecular basis for the higher propensity of Neu5Gc over Neu5Ac in resisting radical oxidation. Further, using human transferrin as a model glycoprotein, we demonstrate the protective role of sialylation against oxidative unfolding. Through a combination of site mapping, enzymatic treatments, and all-ion unfolding ion mobility-mass spectrometry, we identify specific protein sialylation patterns and structural motifs that are crucial for maintaining structural stability under oxidative stress. Our findings provide unprecedented insights into the intricate interplay between sialylation and oxidative stress, highlighting the importance of sialylation in stabilizing protein conformations under various oxidative stresses.
唾液酰化保护糖蛋白免受氧化应激:唾液酸氧化和结构稳定性的机制见解
唾液酰化是一种重要但不稳定的蛋白质修饰,它在调节蛋白质结构、功能和稳定性方面的作用越来越被人们所认识。虽然氧化应激对蛋白质完整性的影响是公认的,但唾液化对这种损伤的保护作用仍然知之甚少。本研究采用微尺度低温等离子体装置模拟细胞氧化应激,产生可控的深层自由基氧化环境。通过对游离唾液酸(Neu5Ac和Neu5Gc)进行时间分辨深度自由基暴露、高分辨率质谱分析和高保真密度泛函数理论计算,我们建立了一个前所未有的氧化途径,揭示了在开环之前独特的逐步侧链氧化。包含100多种氧化中间体的全面自由基氧化图谱为Neu5Gc比Neu5Ac具有更高的抗自由基氧化倾向提供了分子基础。此外,使用人转铁蛋白作为模型糖蛋白,我们证明了唾液化对氧化展开的保护作用。通过位点定位、酶处理和全离子展开离子迁移-质谱的结合,我们确定了特定的蛋白质唾液化模式和结构基序,这些模式和结构基序对于维持氧化应激下的结构稳定性至关重要。我们的发现为唾液酰化和氧化应激之间复杂的相互作用提供了前所未有的见解,强调了唾液酰化在各种氧化应激下稳定蛋白质构象的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


