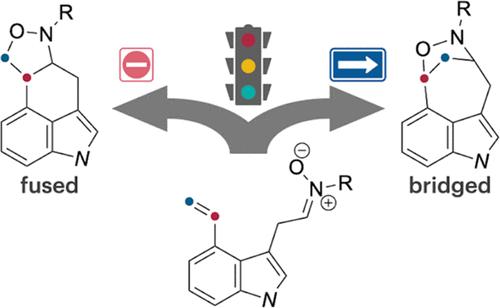
Bridged-Type Selective Intramolecular Nitrone–Alkene Cycloaddition: Computational Chemistry-Inspired Regioselectivity Control
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A regioselective intramolecular nitrone–alkene cycloaddition for synthesizing oxazabicyclo ring-fused indoles is reported. Computational studies guided the development of optimal conditions using In(OTf)3 as a Lewis acidic reagent. This method demonstrates a broad substrate scope, forming seven- and eight-membered carbocycles with various substituents, and provides a versatile route to complex nitrogen-containing scaffolds with potential applications in medicinal chemistry and the total synthesis of biologically active compounds.
桥式选择性分子内硝基-烯烃环加成:计算化学启发的区域选择性控制
报道了一种区域选择性的分子内硝基烯烃环加成法合成恶扎比环熔合吲哚。计算研究指导了使用In(OTf)3作为刘易斯酸性试剂的最佳条件的开发。该方法显示出广泛的底物范围,形成具有各种取代基的七元和八元碳环,为复杂的含氮支架提供了一条多功能途径,在药物化学和生物活性化合物的全合成方面具有潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


