High-Energy, High-Power Sodium-Ion Batteries from a Layered Organic Cathode
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
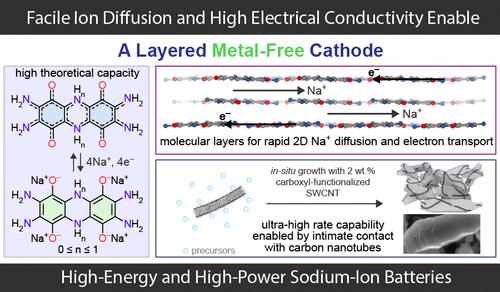
Sodium-ion batteries (SIBs) attract significant attention due to their potential as an alternative energy storage solution, yet challenges persist due to the limited energy density of existing cathode materials. In principle, redox-active organic materials can tackle this challenge because of their high theoretical energy densities. However, electrode-level energy densities of organic electrodes are compromised due to their poor electron/ion transport and severe dissolution. Here, we report the use of a low-bandgap, conductive, and highly insoluble layered metal-free cathode material for SIBs. It exhibits a high theoretical capacity of 355 mAh g–1 per formula unit, enabled by a four-electron redox process, and achieves an electrode-level energy density of 606 Wh kg–1electrode (90 wt % active material) along with excellent cycling stability. It allows for facile two-dimensional Na+ diffusion, which enables a high intrinsic rate capability. Growth of the active cathode material in the presence of as little as 2 wt % carboxyl-functionalized carbon nanotubes improves charge transport and charge transfer kinetics and further enhances the power performance. Altogether, these allow the construction of SIB cells built from an affordable, sustainable organic small molecule, which provide a cathode energy density of 472 Wh kg–1electrode when charging/discharging in 90 s and a top specific power of 31.6 kW kg–1electrode.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


