Metal-N4-Functionalized Graphene as Highly Active Catalysts for C–N Bond Formation in Electrochemical Urea Synthesis
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
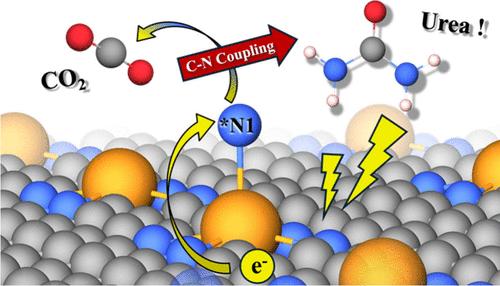
Urea is a vital nitrogen-based fertilizer, traditionally produced through energy-intensive processes that consume significant resources and emit substantial CO2. The electrochemical coreduction of CO2 and NO3–/NO2– offers a sustainable alternative, but efficient catalysts are needed to drive this reaction. In this study, density functional theory calculations combined with a constant electrode potential model were employed to investigate the initial C–N bond formation in urea synthesis via the coreduction of CO2 and NO3–. The reaction was driven by a CuN4 moiety embedded in graphene (gCuN4), a single-atom catalyst (SAC). Despite the common belief that SACs have a limited ability to facilitate C–N coupling due to the lack of adjacent active sites, our findings show that gCuN4 can efficiently promote this reaction via coupling of surface-bound *N1 intermediates, generated from NO3– reduction, with CO2 through the Eley–Rideal mechanism. The calculated free energy barriers are near zero at the experimentally relevant potential of U = −1.0 VSHE. The facile C–N coupling kinetics are retained when Cu is replaced with other first-row transition metals. Surprisingly, the electron dynamics analysis revealed that in most C–N coupling reactions, one of the two electrons forming the C–N bond originates from the graphene support, underscoring its critical role. Additionally, the high reactivity may be due to the use of high-energy electrons from the graphene support and/or nitrogen in the *N1 intermediates rather than relying on the inert Cu–N bond electrons to form C–N bonds. Strategies to enhance C–N bond formation as well as methods to preserve the highly active single-atom state are discussed. These insights will contribute to the development of efficient and durable catalysts for sustainable urea synthesis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


