Rhodium-Catalyzed Allylic Amination for the Enantioselective Synthesis of Tertiary β-Fluoroamines
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The utilization of β-fluoroamines as pharmaceutical components for drug development has attracted a considerable amount of interest. However, direct access to tertiary β-fluoroamines is challenging. We herein report the rhodium-catalyzed asymmetric amination of tertiary allylic trichloroacetimidates with anilines and cyclic aliphatic amines to access tertiary β-fluoroamines, where the α-carbon atom is bonded to four different substituents, in good yield with high levels of enantioselectivity.
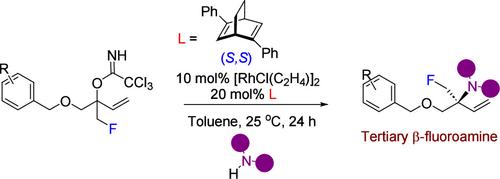
铑催化烯丙基胺化对映选择性合成叔β-氟胺
利用β-氟胺作为药物开发的药物成分已经引起了相当大的兴趣。然而,直接获得叔β-氟胺具有挑战性。本文报道了铑催化的叔烯丙基三氯乙酸酯与苯胺和环脂肪胺的不对称胺化反应,得到叔β-氟胺,其中α-碳原子与四个不同的取代基结合,收率高,对映选择性高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


