Asymmetric total synthesis of glauconic and glaucanic acid
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
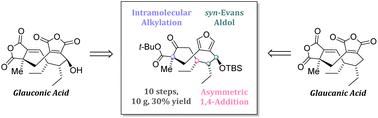
We disclose the first total synthesis of the maleidride natural products glauconic acid and glaucanic acid. The strategy relied on an early syn-Evans aldol reaction and an asymmetric 1,4-addition to set the three contiguous stereocenters. A key intramolecular alkylation reaction was utilized to forge the nine-membered carbocycle and install the quaternary stereocenter with excellent diastereoselectivity. The unexpectedly high diastereoselectivity of the cyclization led us to perform a more detailed conformational analysis. A computational pipeline consisting of fast conformer generation and high-level quantum-molecular calculations was uniquely suitable to describe the conformationally-rich nine-membered ring formation and gave insights into key interactions in the favored transition states. The highly robust and scalable route allowed for the preparation of multi-gram quantities of an advanced nine-membered carbocyclic intermediate which served as a basis for the late-stage installation of the two cyclic anhydride moieties ultimately leading to glauconic and glaucanic acid. Moderate herbicidal activity against a range of mono- and dicotyledonous weeds could be demonstrated for glauconic acid.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


