High-Pressure Effects on an Octa-Hydrated Curium Complex: An Experimental and Theoretical Investigation
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
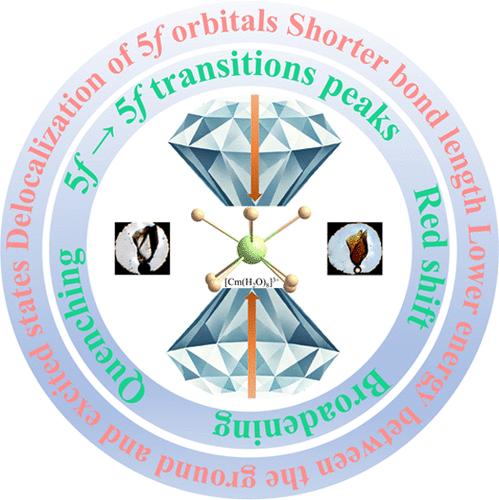
An octa-hydrated curium compound [Cm(H2O)8](Hdtp)(dtp)·H2O (Cm1, H2dtp = 2,3-di(tetrazol-5-yl)pyrazine) along with its lanthanide analogues [Ln(H2O)8](Hdtp)(dtp)·H2O (Ln1, Ln3+ = La3+–Nd3+, Sm3+–Lu3+) were synthesized and characterized using single crystal X-ray diffraction and spectroscopic methods. Bond length analysis of VIIICm(III)–OH2 (where VIII refers to the coordination number) was compared to VIIILn(III)–OH2 (Ln3+ = Nd3+ and Sm3+), indicating similar VIIIM(III)–OH2 bond lengths because of their similar eight-coordinate ionic radii of these VIIIM(III) cations. Owing to the reduced coordination number, the VIIICm(III)–OH2 bond lengths were shorter than previously reported IXCm(III)–OH2 bonds in [Cm(H2O)9](CF3SO3)3. The octa-aquo complexes were also characterized by solid-state UV-vis-NIR spectroscopy in addition to variable-temperature and variable-pressure photoluminescence. Variable-pressure absorption spectra of Cm1 were compared with Ln1 and show that the Cm(III) f → f transitions have a stronger dependence on pressure than that observed in Ln1 (Ln3+ = Nd3+ and Sm3+). The experimental and computational analyses reveal that the monotonic decrease in the computed energy difference between the ground state and the first excited state corresponds to the observed red shift of the photoluminescence peak. This is accompanied by a gradual reduction in the average Cm(III)–OH2 bond length and a delocalization of spin densities, alongside an intensified interaction involving the 5f orbitals under increasing pressure. These changes accommodate the new geometry and collectively modify the energy landscape, resulting in peak broadening and quenching.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


