SLFN11-mediated ribosome biogenesis impairment induces TP53-independent apoptosis
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
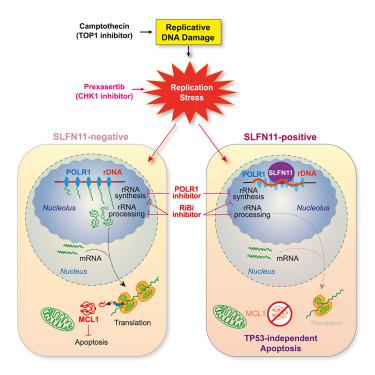
Impairment of ribosome biogenesis (RiBi) triggered by inhibition of ribosomal RNA (rRNA) synthesis and processing leads to various biological effects. We report that Schlafen 11 (SLFN11) induces TP53-independent apoptosis through RiBi impairment. Upon replication stress, SLFN11 inhibits rRNA synthesis with RNA polymerase I accumulation and increased chromatin accessibility in the ribosomal DNA (rDNA) genes. SLFN11-dependent RiBi impairment preferentially depletes short-lived proteins, particularly MCL1, leading to apoptosis in response to replication stress. SLFN11’s Walker B motif (E669), DNA-binding site (K652), dephosphorylation site for single-strand DNA binding (S753), and RNase sites (E209/E214) are all required for the SLFN11-mediated RiBi impairment. Comparable effects were obtained with direct RNA polymerase I inhibitors and other RiBi inhibitory conditions regardless of SLFN11. These findings were extended across 34 diverse human cancer cell lines. Thus, we demonstrate that RiBi impairment is a robust inactivator of MCL1 and an additional proapoptotic mechanism by which SLFN11 sensitizes cancer cells to chemotherapeutic agents.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


