Molecular Evolution of Target Organosulfur Enables High-Performance Aqueous Zinc Batteries
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
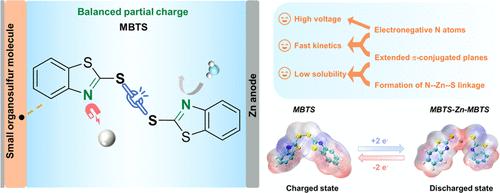
Organic cathode materials for aqueous zinc-ion batteries (AZIBs) have garnered significant attention due to their environmental friendliness and structurally customizable nature. However, the low voltage, sluggish redox kinetics, and high solubility of most n-type cathode materials hinder their wide deployment. To overcome these challenges, through molecular evolution, we rationally select a cheap industrial material, organodisulfide 2,2′-dithiobis (benzothiazole) (MBTS), as an n-type cathode material for AZIBs. Due to the presence of N-containing benzothiazole rings, the dissociation energy of the sulfur−sulfur (S–S) bond is reduced, substantially enhancing the discharge voltage and improving the reaction kinetics. They regulate the π-conjugated plane to achieve a low solubility and fast charge transfer. Moreover, density functional theory (DFT) calculations elucidate the synergistic effect between adjacent active sites and Zn2+ storage reactions, revealing that the formation of weak coordination bonds between N and Zn atoms (N–Zn–S bond) reduces the solubility of the discharge product. Molecular evolution has led to the fast reaction kinetics of zinc ion storage, thus achieving a high performance under high mass loading. At a current density of 0.05 A g–1, MBTS exhibits an average discharge voltage of 1.02 V, with a mere overpotential of 100 mV, and delivers a high specific capacity of 153.6 mAh g–1. The assembled pouch cell demonstrates an excellent rate capability of 124.4 mAh g–1 at 1 A g–1 and displays a stable cycle life after 200 cycles with 96.8% capacity retention. Remarkably, MBTS maintains high specific capacity and favorable cycle stability under various ultrahigh loadings, up to 18.2 mg cm–2. The findings provide substantial guidance for practical applications of organic electrode materials in AZIBs.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


