Impact of Size and Substitution Isomerism in Polycyclic Aromatic-Substituted Trialkoxysilanes on the Formation of Softenable Polysilsesquioxanes
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
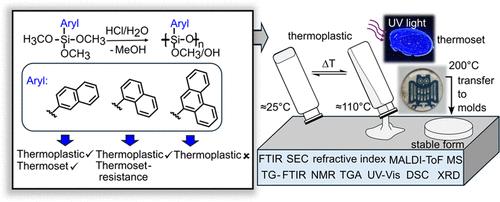
Polyphenylsilsesquioxanes are known to form glassy materials that can reversibly soften when heated above their glass transition temperature, with irreversible curing occurring upon a further temperature increase. In this study, the effects of the size and isomerism of polycyclic aromatic groups on the synthesis and structure of polysilsesquioxanes are investigated, with a focus on their thermoplastic and thermoset properties. Polysilsesquioxanes were synthesized by acid-catalyzed polycondensation using 1-naphthyl, 2-naphthyl, and 9-phenanthrenyltrimethoxysilanes. Characterization techniques, including spectroscopy, thermal analysis, mass determination, and powder X-ray diffraction, showed that steric hindrance by the aromatic groups significantly affects the degree of condensation, the formation of OH groups, and the nature of intra- or intermolecular condensation. Bulky phenanthrenyl groups hinder chain mobility and prevent detectable flow behavior, while 1- and 2-naphthyl groups enable the formation of thermoplastic materials with reversible softening. Notably, 2-naphthylsilsesquioxane undergoes irreversible curing at 200 °C, whereas 1-naphthylsilsesquioxane resists this transition. The incorporation of both polycyclic substituents not only preserves the characteristic thermoplastic behavior of melting gels but also introduces additional properties, such as fluorescence, a high thermal stability up to 460 °C and a high refractive index of 1.61, enhancing the potential of these materials for optical applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


