Elucidating the Dynamic Changes in the Mechanism of the Potential-Dependent Alkaline Hydrogen Evolution Reaction on Platinum
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
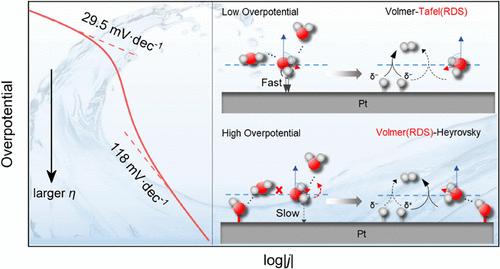
Platinum is widely regarded as the most efficient catalyst for the hydrogen evolution reaction (HER). However, as the overpotential increases, the kinetics of the HER significantly declines and the mechanism exhibits potential-dependent behavior. Through a combination of theoretical simulations and experimental testing, we investigated the changes in the HER mechanism and the underlying kinetic reasons within the kinetic control potential region. The results revealed that at a low cathodic overpotential, the HER follows the Volmer–Tafel mechanism at the Pt(111)/water interface, while at a high cathodic overpotential, it follows the Volmer–Heyrovsky mechanism. The transition and shift in the rate-determining step from the Tafel step to the Volmer step are attributed to the reduced density of active sites and the accumulation of OH generated from water dissociation. Excessive accumulation of OH can promote the desorption of H2 but can also raise the energy barrier of the Volmer step. This occurs because it weakens the adsorption of species and disrupts the orientation of interfacial water on the Pt(111) surface, thus hindering the HER. These findings clarify the significant role of local OH enrichment and its effect on interfacial water in modulating the HER mechanism and enhancing HER kinetics under kinetic control conditions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


