Hydrogen Induced Phase Transition in TiZrNbHfV1–xTax High Entropy Alloys
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
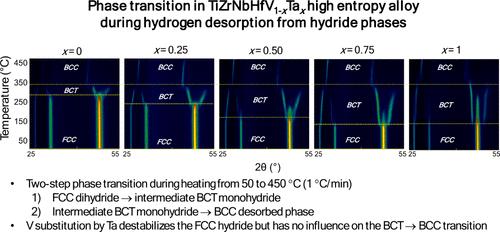
Two extensively researched high-entropy alloys (HEAs) TiZrNbHfV and TiZrNbHfTa have been reported for their high hydrogen capacities yet have shown different behaviors with regard to hydrogen sorption properties. The first composition reacts with hydrogen within a one-step reaction mechanism associated with a phase transition from an initial body-centered cubic (BCC) lattice to body-centered tetragonal (BCT) dihydride phase while the latter alloy undergoes a two-step process from an initial BCC lattice to an intermediate BCT monohydride and finally to a face-centered cubic (FCC) dihydride. For deeper understanding of this change with the substitution of isoelectronic elements, the TiZrNbHfV1–xTax (where x = 0, 0.25, 0.5, 0.75, and 1) alloys have been investigated. The alloys were successfully synthesized using electric arc-melting, resulting in BCC lattices. Rapid hydrogen absorption kinetics were observed at 300 °C, achieving full capacity (2.0 H/M) in around 2 min under 55 bar. In situ neutron diffraction and thermal-desorption spectroscopy demonstrated a two-step phase transition during hydrogen desorption, transitioning from an FCC dihydride to an intermediate BCT monohydride, and finally to a BCC structure. The substitution of V by Ta was found to destabilize the FCC hydride phase, reducing the desorption onset temperature. These findings underscore the potential for tuning hydrogen storage properties through alloy composition, providing insights into the design of advanced HEAs for energy storage applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


