Durvalumab, Tremelimumab, and Platinum Chemotherapy in EGFR Mutation–Positive NSCLC: An Open-Label Phase 2 Trial (ILLUMINATE)
IF 3.5
Q2 ONCOLOGY
引用次数: 0
Abstract
Introduction
EGFR-mutant NSCLC is associated with low mutation burden and low levels of PD-L1 expression. We conducted a phase 2 trial to determine the efficacy of durvalumab, tremelimumab, and platinum-pemetrexed in EGFR-mutant NSCLC after progression with EGFR tyrosine kinase inhibitors (TKIs).
Methods
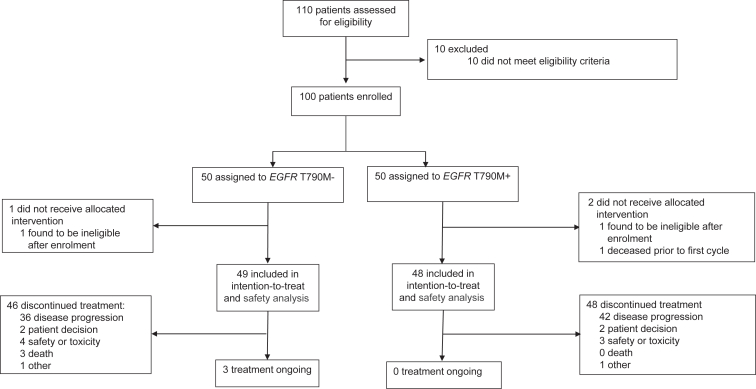
Participants were treated with induction durvalumab, tremelimumab, and platinum-pemetrexed, followed by durvalumab-pemetrexed maintenance. Participants were divided into two cohorts: (1) EGFR exon 20 T790M negative (T790M−, progressing on either first-line osimertinib, or on a single line of first/second generation TKI), and (2) T790M positive (T790M+, progressing on greater than or equal to 1 lines of TKI, including osimertinib). The primary endpoint was the confirmed objective response rate (ORR) assessed by the investigators. Progression-free survival and safety were secondary outcomes.
Results
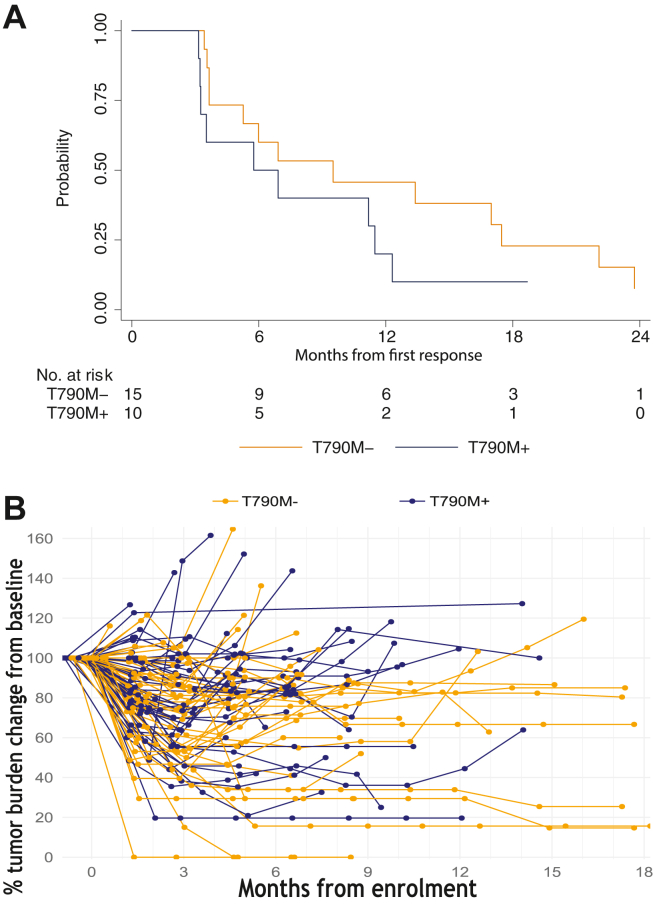
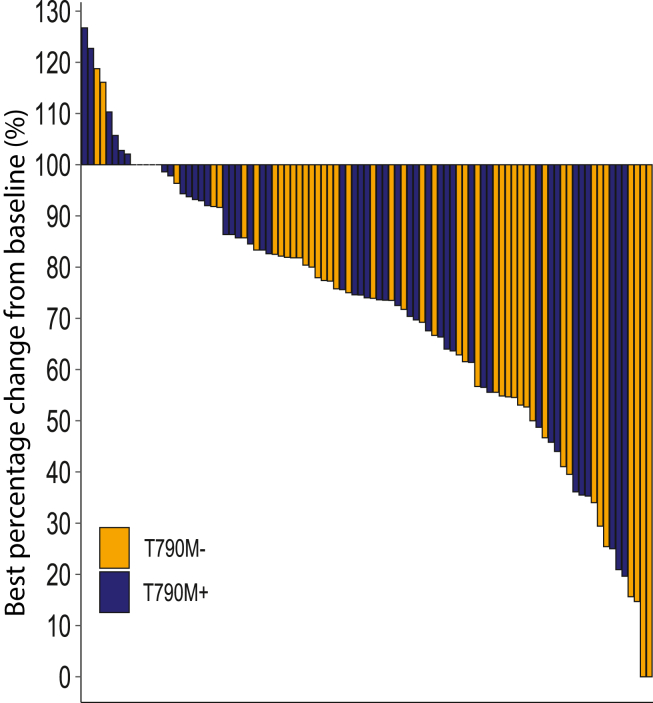
One hundred participants from Australia and Taiwan were enrolled. Median follow-up was 26 months with 88% and 96% experiencing progression events for T790M− and T790M+, respectively. The ORR for T790M− was 31% (95% confidence interval: 20–45), including two complete responses. The ORR for T790M+ was 21% (95% confidence interval: 12–34). Median durations of response were 9.5 months and 6.3 months for T790M− and T790M+, respectively; median progression-free survival rates were 6.5 months and 4.9 months, respectively. For T790M−, ORR was 27% for 50% or higher PD-L1 (n = 22) and 0% for less than 50% PD-L1 (n = 10), respectively. For T790M+, ORR was 17% for 50% or higher PD-L1 (n = 24). The safety profile was consistent with previous reports.
Conclusions
Durvalumab, tremelimumab, and platinum-pemetrexed had modest anti-tumor activity in EGFR-mutant NSCLC after progression on TKI. The T790M− cohort had higher ORR and a longer duration of response. Immune adverse events were not increased with tremelimumab. The clinical registration number of this trial is NCT03994393.
Durvalumab, Tremelimumab和铂化疗治疗EGFR突变阳性NSCLC:一项开放标签2期试验(ILLUMINATE)。
egfr突变型NSCLC与低突变负荷和低水平PD-L1表达相关。我们进行了一项2期试验,以确定durvalumab、tremelimumab和铂-培美曲塞在EGFR酪氨酸激酶抑制剂(TKIs)进展后的EGFR突变型NSCLC中的疗效。方法:参与者接受诱导杜伐单抗、tremelimumab和铂-培美曲塞治疗,随后进行杜伐单抗-培美曲塞维持治疗。参与者被分为两组:(1)EGFR外显子20 T790M阴性(T790M-,一线奥西替尼治疗进展,或一线/二代TKI单线治疗进展);(2)T790M阳性(T790M+,包括奥西替尼在内的1线以上TKI治疗进展)。主要终点是研究人员评估的确定的客观缓解率(ORR)。无进展生存期和安全性是次要结局。结果:来自澳大利亚和台湾的100名参与者入组。中位随访时间为26个月,T790M-组和T790M+组分别有88%和96%出现进展事件。T790M-的ORR为31%(95%可信区间:20-45),包括两个完全应答。T790M+的ORR为21%(95%可信区间:12-34)。T790M-组和T790M+组的中位缓解持续时间分别为9.5个月和6.3个月;中位无进展生存期分别为6.5个月和4.9个月。对于T790M-, 50%及以上PD-L1的ORR为27% (n = 22), 50%及以下PD-L1的ORR为0% (n = 10)。对于T790M+, PD-L1≥50%的患者,ORR为17% (n = 24)。安全概况与以前的报告一致。结论:Durvalumab, tremelimumab和铂-培美曲塞在TKI进展后的egfr突变型NSCLC中具有适度的抗肿瘤活性。T790M组有更高的ORR和更长的反应持续时间。免疫不良事件使用tremelimumab未增加。本试验临床注册号为NCT03994393。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


