Chiral Primary Amine-Catalyzed Asymmetric Photochemical Reactions of Pyridotriazoles with Boronic Acids to Access Triarylmethanes
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Imine-containing azaarene-based triarylmethanes are vital molecular motifs that are prevalent in a wide array of bioactive compounds. Recognizing the limitations of current synthetic methodologies─marked by a scarcity of examples and difficulties in flexible functional group modulation─we have developed an efficient and modular asymmetric photochemical strategy employing pyridotriazoles and boronic acids as substrates. Utilizing novel chiral diamine-derived pyrroles and primary amines as catalysts, we successfully synthesized a diverse range of triarylmethanes with high yields and excellent enantioselectivities. This method not only exhibits a broad substrate scope and outstanding functional group tolerance but also enables the precise synthesis of deuterated derivatives using inexpensive D2O as the deuterium source. Mechanistic studies reveal that an unusual 1,4-boron shift is a critical step in generating the boronated enamine intermediate, while also shedding light on the potential enantiocontrol mechanisms facilitated by the chiral catalyst.
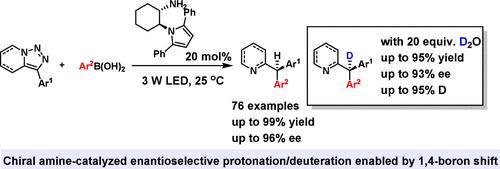
手性伯胺催化吡哆三唑与硼酸合成三芳基甲烷的不对称光化学反应
含亚胺的氮杂环芳烃基三芳基甲烷是广泛存在于多种生物活性化合物中的重要分子基序。认识到当前合成方法的局限性──以缺乏实例和柔性官能团调制的困难为特征──我们开发了一种采用吡哆三唑和硼酸作为底物的高效模块化不对称光化学策略。利用新型手性二胺衍生吡咯和伯胺作为催化剂,成功合成了多种产率高、对映选择性好的三芳基甲烷。该方法不仅具有广泛的底物范围和出色的官能团耐受性,而且可以使用廉价的D2O作为氘源精确合成氘化衍生物。机理研究表明,不寻常的1,4-硼移位是生成硼化enamine中间体的关键步骤,同时也揭示了手性催化剂促进的潜在对映控制机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


