Unveiling New Reactivities in Complex Mixtures: Synthesis of Tricyclic Pyridinium Derivatives
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
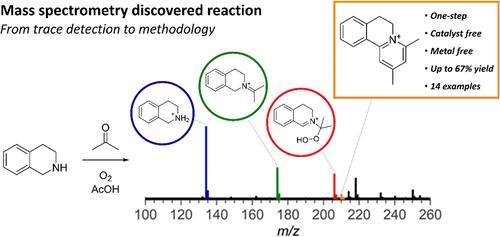
The discovery of new transformations drives the development of synthetic organic chemistry. While the main goal of synthetic chemists is to obtain the maximum yield of a desired product with minimal side product formation, meticulous characterization of the latter offers an opportunity for discovering new reaction pathways, alternative mechanisms, and new products. Herein, we present a case study on the discovery and development of a new chemical transformation using online mass spectrometry. This highly sensitive method enabled the discovery of a new reaction pathway in a catalyst-free cross-dehydrogenative coupling of 1,2,3,4-tetrahydroisoquinoline with acetone via peroxide intermediate, ultimately yielding a tricyclic pyridinium compound. Mass spectrometry was instrumental in detecting and identifying the structure of the pyridinium compound, initially formed as a trace byproduct, which allowed us to develop a general methodology for its exclusive formation.
揭示复杂混合物中的新反应性:三环吡啶衍生物的合成
新转化的发现推动了合成有机化学的发展。虽然合成化学家的主要目标是在最少的副产物形成的情况下获得所需产物的最大产量,但对后者的细致表征为发现新的反应途径、替代机制和新产物提供了机会。在此,我们提出了一个使用在线质谱法发现和开发新的化学转化的案例研究。这种高灵敏度的方法使1,2,3,4-四氢异喹啉与丙酮通过过氧化物中间体进行无催化剂交叉脱氢偶联的新反应途径得以发现,最终生成三环吡啶化合物。质谱法是检测和鉴定吡啶化合物结构的工具,最初是作为一种痕量副产品形成的,这使我们能够为其独特的形成开发一种通用方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


