Cobalt- and Nickel-Doped WSe2 as Efficient Electrocatalysts for Water Splitting and as Cathodes in Hydrogen Evolution Reaction Proton Exchange Membrane Water Electrolysis
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
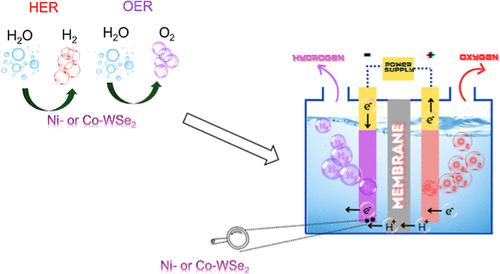
Efficient electrocatalysts are vital for advancing sustainable fuel cell technology, and the use of affordable alternatives that enhance the reaction kinetics is key to progress. Although, tungsten diselenide (WSe2) is promising for electrocatalysis, it is not fully explored, especially in oxygen evolution and in applications such as polymer electrolyte membrane water electrolyzer. In this work, we use a simple approach to dope WSe2 with cobalt or nickel atoms. Both Co– and Ni–WSe2 exhibit excellent oxygen evolution reaction activity, with overpotentials of 370 and 400 mV at 10 mA/cm2, only 90 and 120 mV higher than those of RuO2, respectively. For hydrogen evolution reaction, the materials register low potentials at −10 mA/cm2, with −0.20 V and −0.22 V vs RHE for Ni– and Co–WSe2, respectively. The effective introduction of heteroatoms causes the retention of coordination vacancies, furnishing active catalytic sites that enhanced electrocatalytic performance, resembling this of noble metals in both activity and charge transfer. Moreover, both doped materials show excellent performance and stability as cathode electrocatalysts in the polymer electrolyte membrane water electrolyzer, with great promise for real-world applications. This study promotes sustainable fuel-cell technology through the development of cost-effective, doped WSe2 electrocatalysts that improve water splitting and hydrogen production efficiency.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


