Rhodium-Catalyzed Homogeneous Asymmetric Hydrogenation of Naphthol Derivatives
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
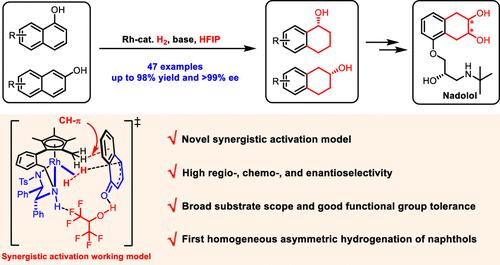
Due to their strong aromaticity and difficulties in chemo-, regio-, and enantioselectivity control, asymmetric hydrogenation of naphthol derivatives to 1,2,3,4-tetrahydronaphthols has remained a long-standing challenge. Herein, we report the first example of homogeneous asymmetric hydrogenation of naphthol derivatives catalyzed by tethered rhodium–diamine catalysts, affording a wide array of optically pure 1,2,3,4-tetrahydronaphthols in high yields with excellent regio-, chemo-, and enantioselectivities (up to 98% yield and >99% ee). Mechanistic studies with experimental and computational approaches reveal that fluorinated solvent 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) plays vital roles in the control of reactivity and selectivity, and 1-naphthol is reduced via a cascade reaction pathway, including dearomative tautomerization, 1,4-hydride addition, and 1,2-hydride addition in sequence. A novel synergistic activation mode was proposed in which HFIP assists a synergistic activation of both the hydrogen molecule and naphthol in the presence of a base, and the in situ-generated fleeting keto tautomer is immediately trapped and reduced by the Rh(III)–H species before it escapes from the solvent cage. This protocol provides a straightforward and practical pathway for the synthesis of key intermediates for several chiral drugs. Particularly, optically pure Nadolol, a drug for the treatment of hypertension, angina pectoris, congestive heart failure, and certain arrhythmias, is enantioselectively synthesized for the first time.
铑催化萘酚衍生物的均相不对称氢化反应
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


