How Do Solvent–Polymer–Surface Interactions Affect the Physisorption of Polymer Chains during Flow-Induced Translocation through Inorganic Oxide Nanochannels?
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
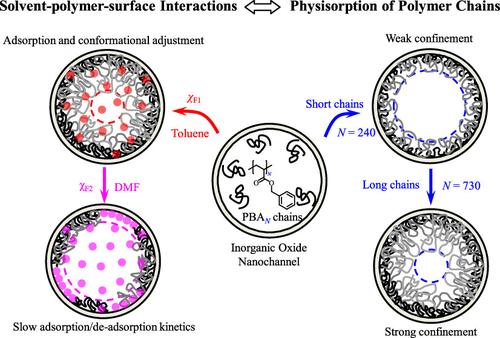
This work aims to explore how polymer–surface–solvent ternary interactions play a synergistical role in affecting the physisorption of PBAN [poly(benzyl acrylate)] chains inside 20 and 100 nm Whatman anodic aluminum oxide nanochannels in flow field by considering the Flory interaction parameter (χF), adsorption parameter (χS), solution concentration (C), and degree of polymerization (N). By using a homemade triple-pump system for in situ monitoring the transmembrane pressure during the solvent switching process, we have found: (1) a combination of χF and χS significantly impacts the adsorption and reversibility of PBAN chains in toluene, ethyl acetate, and tetrahydrofuran, and an extremely slow kinetics process is revealed in dimethylformamide; (2) the adsorption kinetic curves for different PBAN chain lengths are all nicely described by the dual exponential fitting including the fast and slow modes, which can be attributed to the fast approaching of whole chain and the slow reorganization of local conformation, respectively, and the conformational reorganization is found to be the most significant in dimethylformamide; (3) a universal two-regime scaling dependence Aoccupy/Atotal ∼ Nγ is observed between the cross-sectional coverage factor (Aoccupy/Atotal) and N, with γ ∼ 0.50 in the weak confinement regime when Aoccupy/Atotal < 0.30 and γ ∼ 1.5 in the strong confinement when Aoccupy/Atotal > 0.30, independent of pore size, chain length, and solvent type, indicating the dominant effect of the crowding effect when Aoccupy/Atotal > 0.30; (4) the adsorption reversibility and desorption efficiency are found to increase with the solvent quality and polarity in 20 and 100 nm systems during the solvent switching process, which provides a method for the regulation of adsorption thickness; (5) an extremely weak dependence of Aoccupy/Atotal ∼ C is observed, which is consistent with Silberberg’s prediction. Our present result provides useful guidance for understanding and comparing the behavior of chain adsorption in the nonidealized and idealized membrane system.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


