Probing London Dispersion in Proton-Bound Onium Ions: Are Alkyl–Alkyl Steric Interactions Reliably Modeled?
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
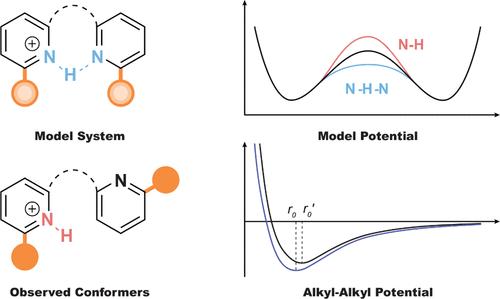
We report spectroscopic and spectrometric experiments that probe the London dispersion interaction between tert-butyl substituents in three series of covalently linked, protonated bis-pyridines in the gas phase. Molecular ions in the three test series, along with several reference molecules for control, were electrosprayed from solution into the gas phase and then probed by infrared multiphoton dissociation spectroscopy and trapped ion mobility spectrometry. The observed N–H stretching frequencies provided an experimental readout diagnostic of the ground-state geometry of each ion, which could be furthermore compared to a second, independent structural readout via the collision cross section. In each of the three series, the strength of a London dispersion interaction could be modulated systematically by a progressive increase in the size of substituents from H to Me to tert-Bu. Parallel to the experimental study, extensive dispersion-corrected density functional theory (DFT-D3BJ) calculations were performed with a range of exchange correlation functionals. A full analysis of the conformational space for the flexible members of the series, and an analysis of the vibrational spectra in the context of a general double-well potential, finds that DFT-D3BJ appears to significantly overbind alkyl–alkyl interactions, specifically interactions between tert-Bu groups, even failing to predict the minimum energy structures reliably in the case of molecules in which London dispersion competes with other noncovalent interactions such as hydrogen bonding.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


