Proton Donors Influence Nitrogen Adsorption in Lithium-Mediated Electrochemical Ammonia Synthesis
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
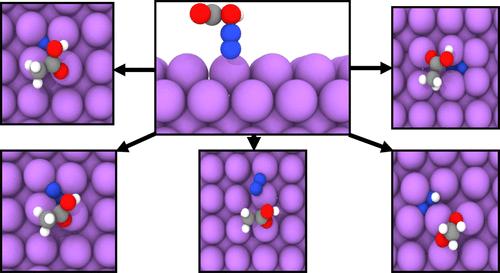
Lithium-mediated electrochemical ammonia synthesis (LiMEAS) has recently shown promise toward efficient electrochemical ammonia production. This process relies on the formation of a lithium nitride film which is subsequently protonated to release ammonia. Designing the electrolyte for this technology requires the selection of a proton donor. In this work, we perform a first-principles analysis to investigate the initial step of nitride formation considering 30 different proton donors (PD). As a baseline, modeling nitrogen on a lithium surface without a PD, we observe that N2 does not spontaneously dissociate on the lithium surface. However, explicitly introducing a PD into the system results in five unique recurring nitrogen configurations on the lithium slab: (1) embedded, (2) adsorbed, (3) standing, (4) buried, and (5) transferred states. We show that these PD-induced states possess an elongated N–N bond and adsorb more strongly on lithium. Using charge analysis, we show that the charge transferred onto these states strongly correlates with the change in their bond length, a crucial parameter for nitrogen dissociation. These results suggest a more involved role of the PD in the initial stages of nitride formation, and motivate greater consideration for their impact on the LiMEAS pathway.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


