Room Temperature Dehydrogenation of Gaseous Methanol over Polycrystalline Gold Triggered and Traced by Oxygen K-edge X-rays
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
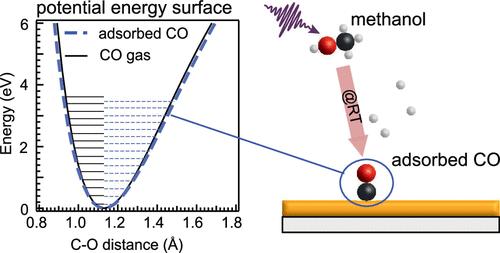
The room temperature conversion of gaseous methanol to carbon monoxide and hydrogen on a polycrystalline Au film at ambient pressure has been triggered and characterized by oxygen K-edge excitation and vibrationally resolved resonant inelastic X-ray scattering. The rate-limiting first methanol dehydrogenation step is driven by ultrafast O–H dissociation and deprotonation of O K-edge excited CH3OH. The Au surface further dehydrogenates the CH3O+ photoradical created by X-rays via electron transfer from the Au surface. With vibrationally resolved resonant inelastic X-ray scattering, we trace the CO molecular potential energy surface along the C–O coordinate. The CO bond softens, and the C–O stretch frequency changes from 2250 to 2065 cm–1 at a CO chemisorption energy of 38–58 kJ/mol. This constitutes weak chemisorption as compared to the transition metals but also stronger bonding than the physisorbed CO species on single-crystal Au surfaces. In liquid methanol, the recombination of the CH3O+ photoradical created by X-rays with protons quenches this conversion.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


