Structural Basis of Ultralow Capacitances at Metal–Nonaqueous Solution Interfaces
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
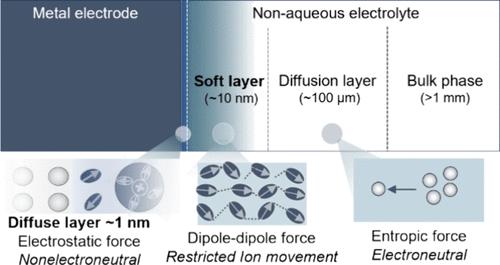
Metal–nonaqueous solution interfaces, a key to many electrochemical technologies, including lithium metal batteries, are much less understood than their aqueous counterparts. Herein, on several metal–nonaqueous solution interfaces, we observe capacitances that are 2 orders of magnitude lower than the usual double-layer capacitance. Combining electrochemical impedance spectroscopy, atomic force microscopy, and physical modeling, we ascribe the ultralow capacitance to an interfacial layer of 10–100 nm above the metal surface. This nanometric layer has a Young’s modulus around 2 MPa, which is much softer than typical solid-electrolyte interphase films. In addition, its AC ionic conductivity is 4-to-5 orders of magnitude lower than that of the bulk electrolyte. The temperature dependencies of the AC ionic conductivity and thickness suggest that the soft layer is formed from metal-mediated, dipole–dipole interactions of the nonaqueous solvent molecules. The observed soft layer opens new avenues of modulating battery performance via rational design of ion transport, (de)solvation, and charge transfer in this interfacial region.
金属-非水溶液界面超低电容的结构基础
金属-非水溶液界面是包括锂金属电池在内的许多电化学技术的关键,但与水相对应的界面相比,人们对其了解甚少。在几个金属-非水溶液界面上,我们观察到电容比通常的双层电容低2个数量级。结合电化学阻抗谱、原子力显微镜和物理模型,我们将超低电容归因于金属表面以上10 - 100nm的界面层。该纳米层的杨氏模量约为2mpa,比典型的固体电解质界面膜柔软得多。此外,其交流离子电导率比本体电解质低4 ~ 5个数量级。交流离子电导率和厚度的温度依赖性表明,软层是由金属介导的非水溶剂分子的偶极子-偶极子相互作用形成的。观察到的软层通过合理设计该界面区域的离子传输、(脱)溶剂化和电荷转移,开辟了调节电池性能的新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


